Online Database of Chemicals from Around the World
| Zhejiang Yangfan New Materials Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8890-2510 8890-2504 8890-2511 | |||
 |
shoufu@shoufuchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2006 | ||||
| Discovery Fine Chemicals Ltd. | UK | Inquire | ||
|---|---|---|---|---|
 |
+44 (1202) 874-517 | |||
 |
pjc@discofinechem.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Shanghai Yingrui Biopharm Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 3358-5366 3466-6753 +86 13311639313 | |||
 |
sales02@shyrchem.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2009 | ||||
| chemBlink standard supplier since 2017 | ||||
| Nanjing Fred Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (25) 8469-6168 | |||
 |
Austin@fredchem.cn | |||
 |
Skype Chat | |||
 |
QQ chat | |||
 |
WeChat: NJFred01 | |||
 |
WhatsApp: +86 17302533743 | |||
| Chemical manufacturer since 2020 | ||||
| chemBlink standard supplier since 2025 | ||||
| Activate Scientific GmbH | Germany | Inquire | ||
|---|---|---|---|---|
 |
+49 (8051) 965-1660 | |||
 |
rschmitt@activate-scientific.com | |||
| Chemical manufacturer | ||||
| Reddy Chemtech Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (404) 483-6270 | |||
 |
sales@reddychemtech.com | |||
| Chemical manufacturer | ||||
| Frontier Specialty Chemicals | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (435) 753-1901 | |||
 |
sales@frontierspecialtychemicals.com | |||
| Chemical manufacturer since 1975 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyrimidine compound >> Thiopyrimidine |
|---|---|
| Name | Ethyl 2-(methylthio)-5-pyrimidinecarboxylate |
| Synonyms | 2-(Methylthio)-5-pyrimidinecarboxylic acid ethyl ester |
| Molecular Structure | 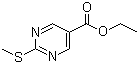 |
| Molecular Formula | C8H10N2O2S |
| Molecular Weight | 198.24 |
| CAS Registry Number | 73781-88-1 |
| EC Number | 640-608-9 |
| SMILES | CCOC(=O)C1=CN=C(N=C1)SC |
| Density | 1.3±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 313.5±15.0 ºC 760 mmHg (Calc.)* |
| Flash point | 143.4±20.4 ºC (Calc.)* |
| Index of refraction | 1.552 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H332-H335 Details |
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P280-P301+P317-P302+P352-P304+P340-P305+P351+P338-P317-P319-P321-P330-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details |
| SDS | Available |
|
Ethyl 2-(methylthio)-5-pyrimidinecarboxylate is a chemical compound belonging to the class of pyrimidine derivatives, a group of organic compounds that contain a six-membered ring structure with two nitrogen atoms at positions 1 and 3. This particular compound features an ethyl ester group (-COOEt) at position 2 of the pyrimidine ring, a methylthio group (-CH3S) at position 5, and a carboxylate functional group (-COO) at position 2. The molecular formula for ethyl 2-(methylthio)-5-pyrimidinecarboxylate is C8H10N2O2S. The compound is typically synthesized through reactions that introduce the methylthio group into the pyrimidine ring, followed by esterification with ethanol to form the ethyl ester. Various synthetic strategies can be employed, such as nucleophilic substitution or direct alkylation reactions, to achieve the desired functionality. Ethyl 2-(methylthio)-5-pyrimidinecarboxylate has demonstrated potential in several areas of medicinal chemistry and organic synthesis. Pyrimidine derivatives, in general, are known for their diverse biological activities and their ability to interact with various biological targets, including enzymes, receptors, and nucleic acids. As a result, ethyl 2-(methylthio)-5-pyrimidinecarboxylate can be considered a valuable building block for the synthesis of bioactive compounds. In particular, the presence of the methylthio group in the structure of this compound can enhance its interaction with specific biological targets, including enzymes involved in metabolic processes. Methylthio groups are known to increase the lipophilicity and metabolic stability of compounds, potentially improving their bioavailability and pharmacokinetic properties. This feature may contribute to the development of pharmaceutical agents with improved efficacy in treating diseases such as cancer, bacterial infections, or inflammatory disorders. The ethyl ester functionality also offers opportunities for further functionalization. The ester group can be hydrolyzed to release the corresponding carboxylic acid, which can then be further modified or used in subsequent reactions to create more complex compounds. This characteristic makes ethyl 2-(methylthio)-5-pyrimidinecarboxylate a versatile intermediate in the synthesis of a variety of other pyrimidine-based molecules with a wide range of potential applications in drug discovery. Beyond its direct applications in medicinal chemistry, ethyl 2-(methylthio)-5-pyrimidinecarboxylate has utility in agrochemical synthesis. Pyrimidine derivatives are often used in the development of herbicides, fungicides, and other pest control agents due to their ability to interact with key enzymes in plant and microbial systems. The compound could potentially be explored as a precursor for the design of new agrochemicals that are effective against a variety of agricultural pests or pathogens, offering the possibility for safer and more environmentally friendly alternatives to conventional pesticides. Additionally, the compound may be used as a precursor in the synthesis of other heterocyclic compounds. The pyrimidine ring structure is a versatile building block in organic chemistry and is widely used in the development of a variety of materials, including polymers, catalysts, and functional materials for electronic devices. Despite its promising applications, further studies are required to fully understand the compound's pharmacological properties and its potential for use in drug development or agrochemical applications. As with all chemical compounds, appropriate safety measures should be followed when handling ethyl 2-(methylthio)-5-pyrimidinecarboxylate to avoid exposure to hazardous substances or reactions. In conclusion, ethyl 2-(methylthio)-5-pyrimidinecarboxylate is a versatile and potentially valuable compound in both medicinal chemistry and agrochemical development. Its functional groups and structural features make it a useful intermediate for synthesizing bioactive compounds, and its potential for further modification increases its utility in drug discovery and industrial applications. The compound's role in the synthesis of pyrimidine-based molecules suggests it could have future relevance in the development of new therapeutic agents and chemical products. |
| Market Analysis Reports |
| List of Reports Available for Ethyl 2-(methylthio)-5-pyrimidinecarboxylate |