Online Database of Chemicals from Around the World
| Zhuhai Jinyou Import & Export Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (311) 8383-6631 | |||
 |
vita@zjpharma.com | |||
| Chemical distributor | ||||
| chemBlink standard supplier since 2006 | ||||
| Capot Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8558-6718 +86 13336195806 | |||
 |
capotchem@gmail.com sales@capotchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Arshine Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (755) 3333-0822 | |||
 |
marketing@arshine.com.cn | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| Rainbow Lab | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (311) 8381-3879 | |||
 |
vita.hao@rainbowlabs.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Shanghai Hohance Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 3111-5312 | |||
 |
info@hohance.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2011 | ||||
| Classification | API >> Antibiotics >> Tetracycline |
|---|---|
| Name | Oxytetracycline |
| Synonyms | Terramycin; 4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide; 5-Hydroxytetracycline |
| Molecular Structure | 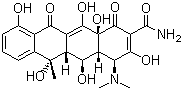 |
| Molecular Formula | C22H24N2O9 |
| Molecular Weight | 460.44 |
| CAS Registry Number | 79-57-2 |
| EC Number | 201-212-8 |
| SMILES | C[C@@]1([C@H]2[C@@H]([C@H]3[C@@H](C(=O)C(=C([C@]3(C(=O)C2=C(C4=C1C=CC=C4O)O)O)O)C(=O)N)N(C)C)O)O |
| Melting point | 183 ºC |
|---|---|
| alpha | -223 º (c=1 0.03N HCl) |
| Water solubility | 0.2 g/L |
| Density | 1.7±0.1 g/cm3, Calc.* |
| Index of Refraction | 1.762, Calc.* |
| Boiling Point | 727.8±60.0 ºC (760 mmHg), Calc.* |
| Flash Point | 394.0±32.9 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |


 GHS07;GHS08;GHS09 Warning Details
GHS07;GHS08;GHS09 Warning Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335-H361-H361d-H362-H410-H411 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P203-P260-P261-P263-P264-P264+P265-P270-P271-P273-P280-P302+P352-P304+P340-P305+P351+P338-P318-P319-P321-P332+P317-P337+P317-P362+P364-P391-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Oxytetracycline is a broad-spectrum antibiotic belonging to the tetracycline class. It was first discovered in the 1940s and is derived from the bacterium Streptomyces rimosus. Oxytetracycline has been widely used in both human and veterinary medicine to treat a variety of bacterial infections, although its use has decreased in recent years due to the development of antibiotic resistance and the availability of newer antibiotics. The mechanism of action of oxytetracycline involves inhibiting bacterial protein synthesis. It achieves this by binding to the 30S ribosomal subunit of the bacterial ribosome, which prevents the attachment of aminoacyl-tRNA to the ribosome, thereby blocking the addition of amino acids to the growing peptide chain. This inhibition of protein synthesis disrupts bacterial growth and replication, ultimately leading to bacterial cell death. Oxytetracycline is considered bacteriostatic, meaning it inhibits bacterial growth rather than directly killing bacteria. However, at higher concentrations, it can exhibit bactericidal effects. Oxytetracycline is effective against a wide range of Gram-positive and Gram-negative bacteria, as well as some atypical organisms such as rickettsiae, chlamydia, and mycoplasma. It is commonly used in the treatment of infections such as respiratory tract infections, urinary tract infections, and certain types of skin infections. In veterinary medicine, oxytetracycline is frequently used to treat infections in livestock, poultry, and fish, as well as in animal feed additives to prevent disease and promote growth. Oxytetracycline is typically administered orally, but it can also be given via intramuscular or intravenous injection. Its bioavailability is generally good when taken orally, although its absorption can be impaired by the presence of calcium, magnesium, and other divalent cations, which form insoluble complexes with the antibiotic. This is why antacids or dairy products should be avoided when taking oxytetracycline. Despite its effectiveness, the use of oxytetracycline is associated with a number of potential side effects. These include gastrointestinal disturbances such as nausea, vomiting, and diarrhea. More serious side effects include liver toxicity, especially with prolonged use, and the development of antibiotic resistance. Additionally, oxytetracycline can cause photosensitivity, leading to an increased risk of sunburn and skin reactions when exposed to sunlight. It may also lead to dental staining, particularly in children under the age of 8, as it can bind to calcium in developing teeth and bones. Oxytetracycline is contraindicated in pregnant women, especially in the second and third trimesters, as it can cross the placenta and affect the development of the fetus. It is also not recommended for use in children under the age of 8 due to the risk of dental staining and bone development issues. In individuals with renal or hepatic impairment, dosage adjustments may be necessary to avoid toxicity. Over the years, the widespread use of oxytetracycline has contributed to the development of bacterial resistance. Many bacteria have developed mechanisms to evade the effects of tetracyclines, including efflux pumps that expel the antibiotic from the bacterial cell and enzymes that inactivate the drug. As a result, the use of oxytetracycline has been restricted in some regions, and alternative antibiotics are often preferred for certain infections. In summary, oxytetracycline is a broad-spectrum antibiotic used to treat a variety of bacterial infections. While it is effective against a wide range of pathogens, its use has decreased due to concerns over antibiotic resistance and side effects, such as gastrointestinal disturbances and dental staining. Its use in both human and veterinary medicine remains important, but it is generally reserved for situations where other antibiotics are less effective or appropriate. References 1979. Evaluation of a new long-acting oxytetracycline formulation against anaplasmosis in colombian cattle. Tropenmedizin und Parasitology, 30(2). URL: https://pubmed.ncbi.nlm.nih.gov/483386 2024. Pharmaceuticals in the Environment and Their Removal in Conventional Wastewater Treatment Plants. Pollutants and Recent Trends in Wastewater Treatment. DOI: 10.1007/978-3-031-62054-6_2 2024. Antibiotics and Antibiotic Resistance Genes in Water. Water Reuse and Unconventional Water Resources. DOI: 10.1007/978-3-031-67739-7_5 |
| Market Analysis Reports |
| List of Reports Available for Oxytetracycline |