Online Database of Chemicals from Around the World
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Nanjing MSN Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (25) 5701-5632 +86 18013328036 | |||
 |
info@msnchem.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2013 | ||||
| chemBlink standard supplier since 2008 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Labseeker Inc | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (858) 750-1632 | |||
 |
marketing@labseeker.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2015 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Hangzhou Molcore Biopharmatech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8102-5280 | |||
 |
sales@molcore.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2010 | ||||
| chemBlink standard supplier since 2017 | ||||
| Shanghai Fuxin Pharmaceutical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 3130-0828 +86 18645121291 | |||
 |
contact@fuxinpharm.com | |||
| Chemical manufacturer since 2016 | ||||
| chemBlink standard supplier since 2018 | ||||
| Classification | Chemical reagent >> Organic reagent >> Ionic liquid |
|---|---|
| Name | 1-Butyl-3-methylimidazolium chloride |
| Molecular Structure | 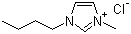 |
| Molecular Formula | C8H15ClN2 |
| Molecular Weight | 174.67 |
| CAS Registry Number | 79917-90-1 |
| EC Number | 627-110-7 |
| SMILES | CCCCN1C=C[N+](=C1)C.[Cl-] |
| Melting point | 73 ºC |
|---|---|
| Flash point | 192 ºC |
| Hazard Symbols |
 GHS06 Danger Details
GHS06 Danger Details | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H301-H315-H319 Details | ||||||||||||||||||||||||||||||||
| Precautionary Statements | P264-P264+P265-P270-P280-P301+P316-P302+P352-P305+P351+P338-P321-P330-P332+P317-P337+P317-P362+P364-P405-P501 Details | ||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Transport Information | UN 2811 | ||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||
|
1-Butyl-3-methylimidazolium chloride (BMIM Cl) is a versatile ionic liquid that has gained prominence in both academic research and industrial applications due to its unique physicochemical properties. BMIM Cl belongs to the family of imidazolyl ionic liquids, which are characterized by their ionic nature and being liquid at relatively low temperatures. The discovery of BMIM Cl is part of a broad development of ionic liquids, which began to receive widespread attention in the late 20th century. Researchers sought new solvents that could overcome the environmental and safety drawbacks of traditional organic solvents. The synthesis of BMIM Cl generally involves the alkylation of 1-methylimidazolium with butyl chloride, resulting in a stable ionic liquid with desirable properties. 1-Butyl-3-methylimidazolium chloride has the molecular formula C8H15ClN2 and has several notable properties: BMIM Cl is stable at high temperatures, making it suitable for a variety of high-temperature processes; its negligible vapor pressure minimizes the release of volatile organic compounds (VOCs) into the environment; the ionic nature of BMIM Cl provides high conductivity, which is very beneficial for electrochemical applications; and it can dissolve a wide range of organic and inorganic substances, making it a versatile solvent. The main application of BMIM Cl is as a green solvent in chemical reactions. Its ability to dissolve a wide range of compounds makes it an ideal choice for organic synthesis, catalysis, and polymerization processes. BMIM Cl can increase reaction rates and selectivity, generally leading to more efficient and environmentally friendly chemical processes. BMIM Cl has been widely studied for its role in biomass processing, especially in the dissolution and pretreatment of cellulose. Its ability to dissolve cellulose and other lignocellulosic materials makes it a valuable tool for the production of biofuels and biochemicals, contributing to the development of sustainable energy. The high ionic conductivity of BMIM Cl makes it an excellent choice for electrochemical devices such as batteries, supercapacitors, and fuel cells. It can improve the performance and lifetime of these devices by providing a stable ionic medium for charge transfer. BMIM Cl is used in the extraction and separation of a wide range of compounds due to its unique solvation properties. It can selectively dissolve specific substances and therefore can be used to purify natural products, metals, and other valuable materials. BMIM Cl can also be used as a catalyst or co-catalyst in a variety of chemical reactions. Its ionic nature can provide a unique catalytic environment that improves the efficiency and yield of chemical processes. This application extends to a range of industries, including pharmaceuticals and petrochemicals. The use of BMIM Cl supports green chemistry initiatives by reducing reliance on hazardous organic solvents. Its low volatility and potential for recycling and reuse make it a more environmentally friendly option for many chemical processes. BMIM Cl is non-flammable and thermally stable, which improves safety in industrial and laboratory settings. Its stability reduces the risk of accidents and contributes to safer chemical handling practices. BMIM Cl's ability to dissolve a wide range of compounds and its application in a variety of fields, from synthesis to electrochemistry, highlights its versatility and importance in modern chemical research and industry. References 2019. Removal of nickel from aqueous solution using synthesized IL/ZnO NPs. Environmental science and pollution research international, 27(1). DOI: 10.1007/s11356-019-07425-8 2012. Pretreatment of sugarcane bagasse by acid-catalysed process in aqueous ionic liquid solutions. Bioresource Technology, 119(1). DOI: 10.1016/j.biortech.2012.06.035 2009. Microwave-assisted synthesis using ionic liquids. Molecular Diversity, 13(4). DOI: 10.1007/s11030-009-9159-3 |
| Market Analysis Reports |
| List of Reports Available for 1-Butyl-3-methylimidazolium chloride |