Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Chemical reagent >> Organic reagent >> Cyanide/nitrile |
|---|---|
| Name | Diphenylacetonitrile |
| Synonyms | 2,2-diphenylacetonitrile |
| Molecular Structure | 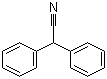 |
| Molecular Formula | C14H11N |
| Molecular Weight | 193.25 |
| CAS Registry Number | 86-29-3 |
| EC Number | 201-662-5 |
| SMILES | C1=CC=C(C=C1)C(C#N)C2=CC=CC=C2 |
| Density | 1.1±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 71 - 73 ºC (Expl.) |
| Boiling point | 322.3 ºC 760 mmHg (Calc.)*, 354.8 ºC (Expl.) |
| Flash point | 151.6±5.7 ºC (Calc.)*, 48.9 ºC (Expl.) |
| Solubility | Insuluble (water) (Expl.) |
| Index of refraction | 1.585 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS06;GHS07 Danger Details
GHS06;GHS07 Danger Details | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H301-H315-H319-H335 Details | ||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P280-P301+P316-P302+P352-P304+P340-P305+P351+P338-P319-P321-P330-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||
|
Diphenylacetonitrile is an organic compound with the molecular formula C14H11N. Structurally, it consists of a central acetonitrile group (–CH(CN)–) flanked by two phenyl rings. This compound belongs to the class of aromatic nitriles and is known for its role as a versatile intermediate in organic synthesis. It is a white to off-white crystalline solid at room temperature and is moderately soluble in common organic solvents such as ethanol, ether, and chloroform. The synthesis of diphenylacetonitrile is typically accomplished through the cyanation of benzophenone using hydrogen cyanide (HCN) or other cyanide sources in the presence of a base. An alternative route involves the condensation of benzyl cyanide with benzene in the presence of a Lewis acid catalyst such as aluminum chloride (AlCl3), following a Friedel–Crafts-type pathway. These procedures highlight the compound’s ease of preparation from readily available starting materials. Diphenylacetonitrile has been utilized extensively as a key intermediate in the synthesis of a variety of fine chemicals, pharmaceuticals, and agrochemicals. One major application lies in its role as a precursor to 2,2-diphenylacetic acid and its derivatives. Hydrolysis of the nitrile group under acidic or basic conditions yields the corresponding carboxylic acid, which can then be further transformed into amides, esters, or alcohols, depending on the desired end product. In medicinal chemistry, diphenylacetonitrile has served as a core building block for the synthesis of nonsteroidal anti-inflammatory drugs (NSAIDs), antihistamines, and central nervous system agents. Its rigid diphenylmethane-like backbone and electron-withdrawing nitrile group allow for modifications that tune biological activity and pharmacokinetic properties. The introduction of various substituents on the phenyl rings, as well as modifications of the nitrile moiety, has led to the development of drug candidates targeting receptors such as histamine H1 and serotonin 5-HT receptors. Beyond pharmaceuticals, diphenylacetonitrile has been studied as a ligand or precursor in coordination chemistry. The nitrile group can participate in metal coordination, offering utility in the preparation of organometallic complexes with potential applications in catalysis. Its chemical reactivity also makes it a useful intermediate in multicomponent reactions and heterocyclic synthesis. The chemical behavior of diphenylacetonitrile is typical of aromatic nitriles. It can undergo nucleophilic attack at the nitrile carbon, as well as electrophilic aromatic substitution reactions on the phenyl rings. Reduction of the nitrile group leads to the corresponding amine, while oxidation can yield imidic acids or other nitrogen-containing functionalities. These properties are leveraged in the stepwise construction of more complex molecules in synthetic organic chemistry. Analytical methods used to characterize diphenylacetonitrile include nuclear magnetic resonance (NMR) spectroscopy, which reveals signals for aromatic protons and the methine proton adjacent to the nitrile. Infrared (IR) spectroscopy shows a strong absorption band near 2,250 cm−1, characteristic of the C≡N stretching vibration. Mass spectrometry (MS) and elemental analysis are also used for confirmation of purity and molecular weight. In terms of handling and safety, diphenylacetonitrile should be stored in a tightly sealed container, protected from moisture and strong oxidizing agents. While it is generally considered to have low acute toxicity, it should be handled with standard laboratory precautions, including the use of gloves, protective eyewear, and appropriate ventilation. In summary, diphenylacetonitrile is a valuable intermediate in organic synthesis with applications in pharmaceuticals, fine chemicals, and coordination chemistry. Its accessible synthesis, chemical reactivity, and structural versatility make it an important compound in both academic and industrial research. References 2014. Direct Transformation of Primary Nitro Compounds into Nitriles with Sodium Dithionite. Synthesis, 46(6). DOI: 10.1055/s-0033-1370874 2013. Heteropolyacid catalyzed click synthesis of 5-substituted 1H-tetrazoles from [bmim]N3 and nitriles under solvent-free conditions. Monatshefte für Chemie - Chemical Monthly, 144(12). DOI: 10.1007/s00706-013-1025-4 2009. The Chemistry of Deprotonated α-Aminonitriles. Synthesis, 2009(12). DOI: 10.1055/s-0029-1216839 |
| Market Analysis Reports |
| List of Reports Available for Diphenylacetonitrile |