Online Database of Chemicals from Around the World
| Wuhan Kemi-works Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (27) 8573-6489 | |||
 |
info@kemiworks.net sales@kemiworks.com | |||
| Chemical manufacturer | ||||
| chemBlink premium supplier since 2011 | ||||
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Organic raw materials >> Aldehyde |
|---|---|
| Name | 2-Chlorobenzaldehyde |
| Synonyms | o-Chlorobenzaldehyde; o-Chlorobenzenecarboxyaldehyde; OCBA |
| Molecular Structure | 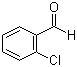 |
| Molecular Formula | C7H5ClO |
| Molecular Weight | 140.57 |
| CAS Registry Number | 89-98-5 |
| EC Number | 201-956-3 |
| SMILES | C1=CC=C(C(=C1)C=O)Cl |
| Density | 1.248 |
|---|---|
| Melting point | 10-11.5 ºC |
| Boiling point | 209-215 ºC |
| Refractive index | 1.565-1.567 |
| Flash point | 87 ºC |
| Water solubility | 0.1-0.5 g/100 mL at 24 ºC |
| Hazard Symbols |


 GHS05;GHS07;GHS09 Danger Details
GHS05;GHS07;GHS09 Danger Details | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H314-H317-H318-H411-H412 Details | ||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P260-P261-P264-P264+P265-P270-P272-P273-P280-P301+P317-P301+P330+P331-P302+P352-P302+P361+P354-P304+P340-P305+P354+P338-P316-P317-P321-P330-P333+P317-P362+P364-P363-P391-P405-P501 Details | ||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| Transport Information | UN 3265 | ||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||
|
2-Chlorobenzaldehyde is an aromatic aldehyde with a chlorine atom substituted at the ortho position of the benzene ring. This chemical compound is known for its use as an intermediate in the synthesis of various organic compounds. Its structure, featuring both an aldehyde group and a chlorine atom, makes it a reactive molecule that finds applications in pharmaceuticals, agrochemicals, and dyes. The discovery of 2-chlorobenzaldehyde can be attributed to advancements in the study of chlorinated aromatic compounds and aldehydes in organic chemistry. The combination of a reactive aldehyde group and an electron-withdrawing chlorine atom enables selective chemical transformations. This compound is especially valued for its ability to undergo condensation and nucleophilic addition reactions, making it a key intermediate in organic synthesis. In the pharmaceutical industry, 2-chlorobenzaldehyde is used to produce active pharmaceutical ingredients (APIs). It serves as a building block in the synthesis of more complex compounds, particularly in the production of sedatives, antihistamines, and other therapeutic agents. The presence of the chlorine atom enhances the bioactivity of the resulting pharmaceutical compounds, improving their efficacy and stability. 2-Chlorobenzaldehyde is also employed in the agrochemical industry, where it is used as an intermediate in the synthesis of pesticides and herbicides. Its ability to undergo transformations into more complex chlorinated compounds provides a pathway for developing chemicals that protect crops from pests while minimizing environmental impact. The reactivity of the aldehyde group allows for the introduction of additional functional groups, increasing the versatility of the compounds produced from 2-chlorobenzaldehyde. Another important application of 2-chlorobenzaldehyde is in the dye and pigment industry. It is used in the production of dyes that are applied to textiles, plastics, and other materials. The chlorine atom contributes to the fastness and brightness of the dyes, making them more resistant to fading under exposure to light and heat. The versatility of 2-chlorobenzaldehyde in various industries, from pharmaceuticals to agrochemicals and dyes, highlights its importance as a valuable intermediate in organic synthesis. Its reactivity and wide range of applications ensure its continued relevance in industrial chemistry. References 2023. Hydrothermal Synthesis of Monoclinic CrVO4 Nanoparticles and Catalytic Ammoxidation of 2-chlorotoluene. Catalysis Letters, 153(3). DOI: 10.1007/s10562-023-04305-2 2023. Continuous catalytic aerobic oxidation of o-chlorotoluene to o-chlorobenzoic acid under slug flow conditions. Journal of Flow Chemistry, 13(3). DOI: 10.1007/s41981-023-00272-2 2023. Diastereoselective Synthesis of 3-[2-Chloro(bromo)phenyl]-2-methyl-2-[(prop-2-yn-1-yl)oxy]oxiranes and 5-[3-Chloro(bromo)phenyl]-2,2,4-trimethyl-4-[(prop-2-yn-1-yl)]-1,3-dioxolane. Russian Journal of Organic Chemistry, 59(5). DOI: 10.1134/s1070428023050032 |
| Market Analysis Reports |
| List of Reports Available for 2-Chlorobenzaldehyde |