Online Database of Chemicals from Around the World
| Zhengzhou Kingorgchem Chemical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (371) 6551-1006 | |||
 |
sales@kingorgchem.com | |||
 |
QQ chat | |||
 |
WeChat: 18625597674 | |||
| Chemical manufacturer since 2015 | ||||
| chemBlink standard supplier since 2016 | ||||
| Classification | Organic raw materials >> Organometallic compound >> Organic iron |
|---|---|
| Name | (2-Bromobenzoyl)ferrocene |
| Molecular Structure | 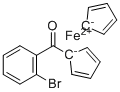 |
| Molecular Formula | C17H13BrFeO |
| Molecular Weight | 369.03 |
| CAS Registry Number | 89670-19-9 |
| SMILES | C1=CC(=C(C=C1)Br)C([C-]2C=CC=C2)=O.[C-]3C=CC=C3.[Fe++] |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H335 Details |
| Precautionary Statements | P280-P305+P351+P338 Details |
| SDS | Available |
|
(2-Bromobenzoyl)ferrocene is an organometallic compound that combines the structural features of ferrocene with a benzoyl group substituted by a bromine atom. This chemical substance has gained prominence in both synthetic chemistry and material science due to its unique properties and versatile applications. The discovery of (2-Bromobenzoyl)ferrocene emerged from efforts to explore the reactivity and utility of ferrocene derivatives in various chemical reactions. Ferrocene, a classic example of a sandwich compound, consists of two cyclopentadienyl rings bound to a central iron atom. The incorporation of a benzoyl group onto one of the cyclopentadienyl rings introduces new functional characteristics to the molecule. The bromine substitution at the 2-position of the benzoyl group further enhances its reactivity, making it a valuable compound for several applications. One of the primary applications of (2-Bromobenzoyl)ferrocene is in the field of organic synthesis. The compound serves as a key intermediate in the synthesis of various ferrocene-based materials and ligands. Its reactivity can be exploited in coupling reactions, where the bromine atom acts as a leaving group, facilitating the formation of new carbon-carbon bonds. This property is particularly useful in the development of complex organic molecules and in the construction of functionalized ferrocene derivatives. In addition to its role in synthesis, (2-Bromobenzoyl)ferrocene is employed in the development of advanced materials. Its ferrocene core provides stability and electronic properties that are advantageous for materials science applications. The bromobenzoyl group can participate in further chemical modifications, leading to the creation of novel materials with tailored properties. These materials can be used in electronic devices, sensors, and as catalysts in various chemical processes. The compound also finds applications in coordination chemistry. The ferrocene moiety can coordinate to metal centers, forming organometallic complexes that exhibit unique reactivity and selectivity. These complexes are useful in catalysis, particularly in reactions that benefit from the stable and tunable nature of ferrocene-based ligands. Overall, (2-Bromobenzoyl)ferrocene represents a significant advancement in the chemistry of ferrocene derivatives. Its discovery has opened up new avenues for research and development in organic synthesis, material science, and coordination chemistry. By combining the robust properties of ferrocene with the reactivity of the bromobenzoyl group, this compound continues to be an important tool for chemists and material scientists. |
| Market Analysis Reports |
| List of Reports Available for (2-Bromobenzoyl)ferrocene |