Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Organic raw materials >> Organometallic compound >> Organomagnesium |
|---|---|
| Name | Cyclohexylmagnesium chloride |
| Molecular Structure | 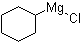 |
| Molecular Formula | C6H11ClMg |
| Molecular Weight | 142.91 |
| CAS Registry Number | 931-51-1 |
| EC Number | 213-237-1 |
| SMILES | C1CC[CH-]CC1.[Mg+2].[Cl-] |
| Density | 0.915 g/mL (25 ºC) (Expl.) |
|---|---|
| Flash point | -10 ºC (closed cup) (Expl.) |
| Hazard Symbols |

 GHS02;GHS05 Danger Details
GHS02;GHS05 Danger Details | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H225-H314-H318 Details | ||||||||||||||||||||
| Precautionary Statements | P210-P233-P240-P241-P242-P243-P260-P264-P264+P265-P280-P301+P330+P331-P302+P361+P354-P303+P361+P353-P304+P340-P305+P354+P338-P316-P317-P321-P363-P370+P378-P403+P235-P405-P501 Details | ||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||
| |||||||||||||||||||||
| Transport Information | UN 3399 4.3/PG 1 | ||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||
|
Cyclohexylmagnesium chloride is a prominent Grignard reagent widely used in organic synthesis. As an organomagnesium halide, it serves as a powerful nucleophile and base, facilitating diverse transformations in academic and industrial chemistry. Cyclohexylmagnesium chloride's discovery is attributed to the foundational work of Victor Grignard in 1900, who developed Grignard reagents and received the Nobel Prize in Chemistry in 1912 for his groundbreaking contributions to synthetic methodology. The reagent is typically prepared by reacting cyclohexyl chloride with magnesium metal in an anhydrous solvent such as diethyl ether or tetrahydrofuran (THF). This exothermic process requires careful control, as the reaction is highly sensitive to moisture and air. The resulting solution of cyclohexylmagnesium chloride is a stable and versatile reagent that finds applications in various chemical reactions. Cyclohexylmagnesium chloride is extensively employed in the formation of carbon-carbon bonds, a cornerstone of organic synthesis. It reacts with electrophiles, such as aldehydes and ketones, to yield secondary and tertiary alcohols, respectively. For instance, its reaction with acetone results in the formation of 2-cyclohexyl-2-propanol. Additionally, it can react with carbon dioxide to produce carboxylic acids or with esters to form tertiary alcohols. In addition to its role in alcohol synthesis, cyclohexylmagnesium chloride is a valuable reagent in the preparation of organometallic intermediates, enabling coupling reactions like the Kumada coupling. These reactions are pivotal in the synthesis of complex organic molecules, including pharmaceuticals, agrochemicals, and specialty materials. Its nucleophilic addition reactions also facilitate the construction of cyclic and polycyclic frameworks, which are common in natural product synthesis. The reagent has notable industrial applications. In the pharmaceutical industry, it is utilized in the synthesis of active pharmaceutical ingredients (APIs) and intermediates. Its ability to selectively introduce cyclohexyl groups is leveraged in designing bioactive molecules with improved pharmacological properties. Furthermore, cyclohexylmagnesium chloride finds use in polymer chemistry, where it acts as an initiator or modifying agent in the synthesis of functionalized polymers. Handling cyclohexylmagnesium chloride requires precautions due to its high reactivity. Exposure to air or moisture can degrade the reagent, necessitating its storage and use under an inert atmosphere. Advances in reagent formulation, including stabilized solutions, have improved its usability and safety in laboratory and industrial settings. Cyclohexylmagnesium chloride exemplifies the utility of Grignard reagents in modern organic synthesis. Its versatility and efficiency in facilitating a broad spectrum of chemical transformations underscore its enduring significance in synthetic chemistry. References 2018. Coupling with Organomagnesium Halides. Science of Synthesis. URL: https://science-of-synthesis.thieme.com/app/text/?id=SD-118-00706 2018. Retaining Alkyl Nucleophile Regiofidelity in Transition-Metal-Mediated Cross-Couplings to Aryl Electrophiles. Synthesis, 50(18). DOI: 10.1055/s-0037-1609941 2016. Consecutive Reaction of Methyleneaziridines with Organomagnesium Chlorides, Organic Bromides, and Phosphonates. Science of Synthesis. URL: https://science-of-synthesis.thieme.com/app/text/?id=SD-130-00139 |
| Market Analysis Reports |
| List of Reports Available for Cyclohexylmagnesium chloride |