Online Database of Chemicals from Around the World
| Zhejiang Yangfan New Materials Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8890-2510 8890-2504 8890-2511 | |||
 |
shoufu@shoufuchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2006 | ||||
| Capot Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8558-6718 +86 13336195806 | |||
 |
capotchem@gmail.com sales@capotchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| Futoh Chemicals Co., Ltd. | Japan | Inquire | ||
|---|---|---|---|---|
 |
+81 (52) 521-8171 | |||
 |
tokoro@futoh.co.jp | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2007 | ||||
| Zhejiang Liaoyuan Pharmaceutical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (576) 8511-3128 | |||
 |
billior@liaoyuan.com.cn | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2008 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| AllessaSyntec GmbH & Co. KG | Germany | Inquire | ||
|---|---|---|---|---|
 |
+49 (69) 3054-7334 | |||
 |
steffen.partzsch@allessasyntec.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Dishman Pharmaceuticals & Chemicals Limited | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (732) 560-4300 | |||
 |
bhaveshoza@dishmangroup.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2011 | ||||
| Classification | Organic raw materials >> Aldehyde |
|---|---|
| Name | 2-Thenaldehyde |
| Synonyms | 2-Formylthiophene; 2-Thiophenecarboxaldehyde; 2-Thiophene carboxaldehyde; Thiophene-2-carbaldehyde |
| Molecular Structure | 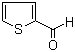 |
| Molecular Formula | C5H4OS |
| Molecular Weight | 112.15 |
| CAS Registry Number | 98-03-3 |
| EC Number | 202-629-8 |
| SMILES | C1=CSC(=C1)C=O |
| Density | 1.2 |
|---|---|
| Boiling point | 198 ºC |
| Refractive index | 1.589-1.592 |
| Flash point | 77 ºC |
| Water solubility | insoluble |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H315-H317-H319-H335 Details | ||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P272-P280-P301+P317-P302+P352-P304+P340-P305+P351+P338-P319-P321-P330-P332+P317-P333+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 2810 | ||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||
|
2-Thenaldehyde, also known as 2-thiophenecarboxaldehyde, is a versatile organic compound with the chemical formula C₇H₆OS. This compound is a derivative of thiophene, where a formyl group is substituted at the 2-position of the thiophene ring. Its discovery and applications span several fields, including organic synthesis, pharmaceuticals, and material science. The discovery of 2-Thenaldehyde is rooted in the study of thiophene derivatives, which began in the late 19th and early 20th centuries. Thiophene itself was first isolated in 1885 by the chemist Victor Meyer, and subsequent research led to the development of various functionalized thiophene derivatives, including 2-Thenaldehyde. The introduction of the formyl group at the 2-position of the thiophene ring imparts distinct reactivity and properties to the compound, making it valuable in various chemical processes. In organic synthesis, 2-Thenaldehyde is utilized as an intermediate in the preparation of various thiophene-based compounds. Its formyl group provides a reactive site for further chemical modifications, allowing chemists to create a range of derivatives with tailored properties. These derivatives find applications in the synthesis of pharmaceuticals, agrochemicals, and other fine chemicals. One notable application of 2-Thenaldehyde is in the pharmaceutical industry, where it serves as a precursor in the synthesis of various therapeutic agents. Its unique thiophene structure contributes to the biological activity of the resulting compounds, making them useful in drug discovery and development. For example, 2-Thenaldehyde has been used in the synthesis of anti-inflammatory agents and antimicrobial compounds. In material science, 2-Thenaldehyde is employed in the development of organic semiconductors and materials for electronic applications. Its ability to form stable conjugated systems with other organic molecules makes it suitable for use in organic light-emitting diodes (OLEDs), organic solar cells, and other electronic devices. The compound's stability and electronic properties are key factors in its suitability for these applications. Overall, 2-Thenaldehyde is an important compound with diverse applications in organic synthesis, pharmaceuticals, and material science. Its discovery and subsequent use highlight its significance in advancing both chemical research and industrial applications. References 2013. Allylation Reactions of Aldehydes with Allylboronates in Aqueous Media: Unique Reactivity and Selectivity that are Only Observed in the Presence of Water. Chemistry - An Asian Journal, 8(9). DOI: 10.1002/asia.201300440 2009. Synthesis and pharmacological evaluation of schiff bases of 4-(2-aminophenyl)-morpholines. Indian Journal of Pharmaceutical Sciences, 71(4). DOI: 10.4103/0250-474x.57292 2008. Novel 3-aroylpyrazolo[5,1-c][1,2,4]benzotriazine 5-oxides 8-substituted, ligands at GABAA/benzodiazepine receptor complex: Synthesis, pharmacological and molecular modeling studies. Bioorganic & Medicinal Chemistry, 16(7). DOI: 10.1016/j.bmc.2008.02.058 |
| Market Analysis Reports |
| List of Reports Available for 2-Thenaldehyde |