Online Database of Chemicals from Around the World
| Suzhou Biosyntech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13921151340 +86 (512) 6300-1269 | |||
 |
sales2@biosyntech-suzhou.com sales@biosyntech-suzhou.com | |||
 |
QQ chat | |||
| Chemical distributor since 2019 | ||||
| chemBlink standard supplier since 2020 | ||||
| Sichuan Taienkang Pharmaceutical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 15102825326 | |||
 |
sales@taienkangpharma.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2020 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Organic raw materials >> Aryl compounds >> Naphthalenes |
|---|---|
| Name | (S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-amine |
| Synonyms | (2S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-amine |
| Molecular Structure | 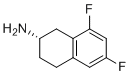 |
| Molecular Formula | C10H11F2N |
| Molecular Weight | 183.20 |
| CAS Registry Number | 1213536-98-1 |
| SMILES | C1CC2=C(C[C@H]1N)C(=CC(=C2)F)F |
| Density | 1.2±0.1 g/cm3, Calc.* |
|---|---|
| Index of Refraction | 1.524, Calc.* |
| Boiling Point | 242.4±40.0 ºC (760 mmHg), Calc.* |
| Flash Point | 122.2±14.4 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
|
(S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-amine is an important compound in pharmaceutical chemistry, primarily recognized for its role as an intermediate in the synthesis of bioactive molecules. The discovery of this compound is linked to the development of fluorinated analogs of tetrahydronaphthalene derivatives, which exhibit enhanced pharmacological properties. Fluorination at the 6 and 8 positions of the naphthalene ring system imparts increased metabolic stability and improved binding affinity in drug candidates. The synthesis of (S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-amine typically begins with the catalytic asymmetric hydrogenation of difluoro-substituted naphthalenes, followed by reductive amination to introduce the amine group at the 2-position. The use of chiral catalysts enables the selective formation of the (S)-enantiomer, which is critical for achieving desired biological activity. This synthetic pathway has been optimized to enhance yield and enantiomeric purity. (S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-amine finds applications in the development of pharmaceutical agents targeting neurological and psychiatric disorders. The compound serves as a key intermediate in the synthesis of serotonin receptor agonists and dopamine modulators. Its structural characteristics contribute to receptor selectivity and potency, making it valuable in the design of treatments for depression, anxiety, and other central nervous system conditions. Research continues to explore the broader application of this compound in medicinal chemistry. Studies indicate potential utility in cancer therapeutics and antiviral drugs, driven by the compound’s ability to modulate critical biological pathways. Its incorporation into drug discovery pipelines underscores the importance of stereochemistry and fluorination in modern pharmaceutical development. References 2017. Chemoenzymatic Route to Chiral Gamma Secretase Inhibitor Synthesis of. Synthesis of chiral gamma secretase inhibitors Synfacts. 2017. DOI: / |
| Market Analysis Reports |
| List of Reports Available for (S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-amine |