Online Database of Chemicals from Around the World
|
CAS: 1435933-84-8 Product: Norfentanyl-d5 Oxalate No suppilers available. |
| Classification | Organic raw materials >> Heterocyclic compound >> Piperidines |
|---|---|
| Name | Norfentanyl-d5 Oxalate |
| Synonyms | Oxalic acid N-(2,3,4,5,6-pentadeuteriophenyl)-N-piperidin-4-ylpropanamide |
| Molecular Structure | 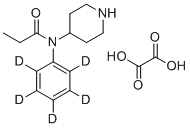 |
| Molecular Formula | C16H17D5N2O5 |
| Molecular Weight | 327.39 |
| CAS Registry Number | 1435933-84-8 |
| EC Number | 806-239-4 |
| SMILES | [2H]C1=C(C(=C(C(=C1[2H])[2H])N(C2CCNCC2)C(=O)CC)[2H])[2H].C(=O)(C(=O)O)O |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H315-H319 Details | ||||||||||||||||||||
| Precautionary Statements | P264-P264+P265-P270-P280-P301+P317-P302+P352-P305+P351+P338-P321-P330-P332+P317-P337+P317-P362+P364-P501 Details | ||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||
| |||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||
|
Norfentanyl-d5 oxalate is a deuterium-labeled derivative of norfentanyl, used primarily as an internal standard in analytical chemistry, particularly in forensic and clinical toxicology. It contains five deuterium atoms that replace five hydrogen atoms in the norfentanyl molecule, providing a precise mass shift while preserving the compound’s chemical and physical properties. This isotopic labeling is critical for high-precision quantitative analyses using techniques such as liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). Norfentanyl itself is the primary inactive metabolite of fentanyl, a synthetic opioid analgesic with high potency at the μ-opioid receptor. After fentanyl administration, it undergoes extensive hepatic metabolism, mainly through N-dealkylation, to form norfentanyl. While norfentanyl lacks significant pharmacological activity, its presence in biological fluids such as urine or plasma serves as a key biomarker for recent fentanyl exposure. Accurate detection of norfentanyl is thus essential for drug testing, therapeutic monitoring, and forensic investigations. The incorporation of five deuterium atoms into the norfentanyl structure creates norfentanyl-d5, which behaves nearly identically to the native compound in chromatographic and ionization processes but can be clearly distinguished by its slightly higher mass-to-charge ratio in a mass spectrometer. The use of norfentanyl-d5 as an internal standard compensates for potential variability during sample preparation and analysis, ensuring accurate quantification of norfentanyl in complex biological matrices. The oxalate salt form of norfentanyl-d5 improves the compound’s stability and facilitates its formulation and handling in laboratory settings. Oxalate salts are often used for amines to produce solid, crystalline forms that are less hygroscopic and more stable than their free base counterparts. This enhances reproducibility and simplifies the preparation of standard solutions used in calibration and quality control procedures. Synthesis of norfentanyl-d5 involves selective deuteration at specific sites of the fentanyl molecule, typically followed by N-dealkylation and salt formation with oxalic acid. The process ensures high isotopic purity and precise positioning of deuterium atoms to minimize isotope exchange and degradation under analysis conditions. Analytical suppliers often characterize the product by nuclear magnetic resonance (NMR) spectroscopy, high-resolution mass spectrometry (HRMS), and chromatographic purity assays to confirm its identity and suitability for laboratory use. Applications of norfentanyl-d5 oxalate are limited to scientific and forensic laboratories. It plays a crucial role in testing protocols for fentanyl and related compounds, which are frequently implicated in opioid overdose cases. The compound is used to validate the performance of analytical methods, establish calibration curves, and monitor procedural consistency across different instruments and batches. Despite its lack of biological activity, norfentanyl-d5 oxalate is handled under controlled conditions due to its close structural relationship with fentanyl and its metabolites. It is not intended for human or veterinary use and is distributed under regulatory oversight to accredited laboratories and institutions. In conclusion, norfentanyl-d5 oxalate is a specialized analytical reagent used to support the accurate detection and quantification of norfentanyl in biological samples. Its role is fundamental in toxicological and forensic workflows aimed at monitoring fentanyl exposure and contributing to public health and safety initiatives related to synthetic opioid use. |
| Market Analysis Reports |
| List of Reports Available for Norfentanyl-d5 Oxalate |