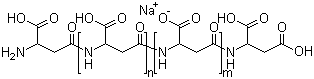Sodium polyaspartic acid, a derivative of aspartic acid, is a synthetic polymer that has gained significant attention for its diverse applications in various fields, including agriculture, medicine, and industrial processes. Its discovery can be traced back to advancements in polymer chemistry during the late 20th century when researchers sought to develop new biocompatible materials. Sodium polyaspartic acid is characterized by its repeating units of aspartic acid linked by peptide bonds, with sodium ions providing solubility and enhancing its functionality in different applications.
The synthesis of sodium polyaspartic acid typically involves the polymerization of aspartic acid using methods such as solution polymerization or ring-opening polymerization. The resulting polymer is a water-soluble, biodegradable compound that exhibits excellent chelating properties due to the presence of carboxyl groups in its structure. This characteristic makes sodium polyaspartic acid a valuable compound in a variety of applications, particularly in agriculture and water treatment.
One of the primary applications of sodium polyaspartic acid is as a biodegradable chelating agent in agriculture. Its ability to bind metal ions effectively helps in nutrient delivery to plants, improving the bioavailability of essential micronutrients like iron, zinc, and manganese. This chelation process enhances plant growth and productivity while reducing the need for chemical fertilizers. Additionally, the use of sodium polyaspartic acid in fertilizers helps to minimize the environmental impact associated with traditional agricultural practices.
In the realm of water treatment, sodium polyaspartic acid has been employed as a scale inhibitor and dispersant. Its chelating properties allow it to bind to calcium and magnesium ions, preventing the formation of scale in pipes and water systems. This application is particularly beneficial in industrial settings where water quality is crucial for maintaining equipment efficiency and longevity. Moreover, its biodegradable nature ensures that it does not pose significant environmental risks when used in water treatment processes.
Sodium polyaspartic acid has also found applications in the biomedical field, where it is used in drug delivery systems and as a stabilizing agent for pharmaceuticals. Its biocompatibility makes it an attractive option for formulating drug carriers that can enhance the solubility and stability of poorly soluble drugs. Furthermore, its ability to form hydrogels enables controlled release of therapeutic agents, providing sustained drug delivery over time. Research continues to explore its potential in developing innovative drug formulations, particularly for targeting cancer and other chronic diseases.
In cosmetics and personal care products, sodium polyaspartic acid is utilized for its moisturizing properties. Its ability to retain water makes it a popular ingredient in skin care formulations, helping to improve hydration and skin texture. As consumer demand for natural and biodegradable ingredients increases, the inclusion of sodium polyaspartic acid in cosmetic formulations aligns with trends towards sustainability and safety in personal care products.
Despite its many advantages, the use of sodium polyaspartic acid requires consideration of its synthesis and application processes to ensure safety and efficacy. Ongoing research focuses on optimizing production methods and exploring new applications to enhance its effectiveness and reduce costs.
In summary, sodium polyaspartic acid is a versatile polymer with significant applications across various fields, including agriculture, water treatment, and biomedicine. Its discovery marked a noteworthy advancement in polymer chemistry, leading to the development of sustainable and biodegradable materials that offer numerous benefits. As research progresses, sodium polyaspartic acid is poised to play an increasingly important role in addressing contemporary challenges in agriculture and environmental sustainability.
|