Online Database of Chemicals from Around the World
| Shanghai ZaiQi Bio-Tech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 5482-4098 | |||
 |
sales1@chemzq.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink standard supplier since 2009 | ||||
| Classification | Organic raw materials >> Carboxylic compounds and derivatives >> Carboxylic esters and their derivatives |
|---|---|
| Name | Methyl 4-fluoro-5-hydroxy-2-methoxybenzoate |
| Molecular Structure | 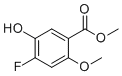 |
| Molecular Formula | C9H9FO4 |
| Molecular Weight | 200.16 |
| CAS Registry Number | 2091274-24-5 |
| SMILES | COC1=CC(=C(C=C1C(=O)OC)O)F |
| Density | 1.3±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 315.8±37.0 ºC 760 mmHg (Calc.)* |
| Flash point | 144.8±26.5 ºC (Calc.)* |
| Index of refraction | 1.517 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H315-H319-H335 Details |
| Precautionary Statements | P261-P305+351+338-P302+352 Details |
| SDS | Available |
|
Methyl 4-fluoro-5-hydroxy-2-methoxybenzoate (CAS 2091274-24-5, formula C₉H₉FO₄) is a substituted aromatic ester combining a fluorine atom, a phenolic hydroxyl, and a methoxy group on a benzoate scaffold. This compound is primarily used as a synthetic intermediate. The hydroxyl (–OH) at the 5-position and the methoxy (–OCH₃) at the 2-position create a functionalized ring system amenable to further derivatization. For example, the phenolic OH can be protected, alkylated, or used in coupling reactions, while the methoxy group contributes to the molecule’s electronic properties and steric profile. The ester moiety (methyl benzoate) provides a site for hydrolysis to the carboxylic acid, or derivatization into amides, acids, or other esters. In medicinal chemistry, such compounds can serve as building blocks for drug-like scaffolds: the fluorine atom can improve metabolic stability or binding interactions, the phenolic OH can participate in hydrogen bonding, and the ester provides a handle for prodrug strategies or further chemical elaboration. Because of these substituents, the molecule offers strategic points for diversification during lead-optimization. Depending on synthetic route, one could prepare this compound by starting from appropriately substituted fluorobenzoic acids. A possible approach: begin with a fluorobenzoic acid bearing substituents at the 4- and 5-positions, methylate the acid to form the methyl ester, then introduce or manipulate the hydroxyl and methoxy groups via electrophilic substitution, demethylation/methylation, or selective functional group interconversion. Protective-group strategies may be needed to manage the reactivity of the phenol and the methoxy in the presence of other functionalizations. From a practical standpoint, handling this ester requires standard precautions: it should be stored in a cool, dry place, protected from light and moisture. The acidic proton on the phenol could make the compound somewhat reactive under strongly basic or nucleophilic conditions, and the ester may hydrolyze under basic aqueous workup if not carefully controlled. Overall, methyl 4-fluoro-5-hydroxy-2-methoxybenzoate is a useful intermediate in the synthesis of advanced aromatic molecules in medicinal and organic chemistry contexts, valued for its multifunctionality and potential for further transformation. References Combinations of RXFP1 modulators and SGLT2 inhibitors. WO-2023237512-A1. Priority date: 2022-06-07. Link RXFP1 modulators for treating resistant hypertension or heart failure with pulmonary hypertension. WO-2023237511-A1. Priority date: 2022-06-07. Link 4-(2-fluoro-4-methoxy-5-3-(((1-methylcyclobutyl)methyl)carbamoyl)bicyclo[2.2.1]heptan-2-yl)carbamoyl)phenoxy-1-methylcyclohexane-1-carboxylic acid derivatives and similar compounds as RXFP1 modulators for the treatment of heart failure. WO-2022122773-A1. Priority date: 2020-12-08. Link |
| Market Analysis Reports |
| List of Reports Available for Methyl 4-fluoro-5-hydroxy-2-methoxybenzoate |