Online Database of Chemicals from Around the World
| Jiangsu Nengyue New Material Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13376001577 | |||
 |
robin@jiangsunengyue.com | |||
 |
WhatsApp: +8613376001577 | |||
| Chemical manufacturer since 2022 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Chemical reagent >> Organic reagent >> Sulfonate / sulfinate |
|---|---|
| Name | Bis(4-(tert-butyl)phenyl)iodonium 2-(trifluoromethyl)benzenesulfonate |
| Molecular Structure | 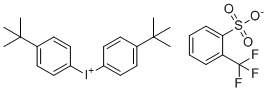 |
| Molecular Formula | C27H30F3IO3S |
| Molecular Weight | 618.49 |
| CAS Registry Number | 229325-98-8 |
| EC Number | 682-856-0 |
| SMILES | CC(C)(C)C1=CC=C(C=C1)[I+]C2=CC=C(C=C2)C(C)(C)C.C1=CC=C(C(=C1)C(F)(F)F)S(=O)(=O)[O-] |
| Hazard Symbols |
 GHS05 Danger Details
GHS05 Danger Details | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H314 Details | ||||||||||||
| Precautionary Statements | P260-P264-P280-P301+P330+P331-P302+P361+P354-P304+P340-P305+P354+P338-P316-P321-P363-P405-P501 Details | ||||||||||||
| Hazard Classification | |||||||||||||
| |||||||||||||
|
Bis(4-(tert-butyl)phenyl)iodonium 2-(trifluoromethyl)benzenesulfonate is a high-performance photoinitiator used in various industrial polymerization processes. This iodonium salt belongs to a class of compounds that generate reactive cationic species upon exposure to UV light, facilitating the polymerization of monomers such as epoxies and vinyl ethers. The molecular structure, characterized by two tert-butylphenyl groups attached to the iodonium cation, enhances its solubility in organic solvents, while the 2-(trifluoromethyl)benzenesulfonate anion plays a stabilizing role, contributing to the compound’s effectiveness in initiating polymerization reactions. The discovery of iodonium salts, including Bis(4-(tert-butyl)phenyl)iodonium 2-(trifluoromethyl)benzenesulfonate, emerged from research aimed at developing photoinitiators for fast and efficient polymerization under UV light. These salts proved to be ideal candidates due to their ability to undergo photodecomposition, generating reactive intermediates that promote cationic polymerization. The inclusion of tert-butyl groups in the phenyl rings increases the photoinitiator’s solubility, making it suitable for diverse applications in UV-curable formulations. This compound is primarily used in UV-curable coatings, adhesives, and inks. In the coatings industry, Bis(4-(tert-butyl)phenyl)iodonium 2-(trifluoromethyl)benzenesulfonate is a key component in the production of protective films for electronic devices, automotive parts, and optical lenses. These coatings provide excellent durability, chemical resistance, and abrasion resistance, all of which are critical for extending the lifespan of treated surfaces. The fast-curing nature of this photoinitiator helps manufacturers increase production efficiency by reducing the time required for materials to harden under UV light. In the field of adhesives, this compound is used in formulations that require rapid curing and strong adhesion. UV-curable adhesives are widely applied in sectors such as electronics, medical devices, and optical components, where high bond strength and precision are essential. Bis(4-(tert-butyl)phenyl)iodonium 2-(trifluoromethyl)benzenesulfonate ensures quick curing times, improving overall processing speeds and reducing energy consumption during manufacturing. In printing technologies, the photoinitiator is an essential component of UV-curable inks. These inks are used in a variety of printing applications, including packaging, labels, and specialty graphics. The ability of this compound to initiate polymerization quickly under UV light results in inks that are smudge-proof, glossy, and highly resistant to fading. The stable nature of the photoinitiator ensures that the inks adhere uniformly to various substrates, including plastics, metals, and glass. The compound’s performance as a photoinitiator is further enhanced by the presence of the 2-(trifluoromethyl)benzenesulfonate anion. This anion contributes to the overall stability of the iodonium salt, ensuring that the compound remains active even under challenging environmental conditions. Its weakly coordinating nature facilitates the rapid release of the iodonium cation during UV exposure, promoting fast polymerization rates. The synthesis of Bis(4-(tert-butyl)phenyl)iodonium 2-(trifluoromethyl)benzenesulfonate typically involves the reaction of iodine(III) derivatives with tert-butyl-substituted phenyl groups, followed by the introduction of the trifluoromethylbenzenesulfonate anion. This method ensures high purity and yields of the photoinitiator, which is then subjected to rigorous testing for its photopolymerization efficiency in different applications. |
| Market Analysis Reports |
| List of Reports Available for Bis(4-(tert-butyl)phenyl)iodonium 2-(trifluoromethyl)benzenesulfonate |