Online Database of Chemicals from Around the World
| Jiangsu Nengyue New Material Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13376001577 | |||
 |
robin@jiangsunengyue.com | |||
 |
WhatsApp: +8613376001577 | |||
| Chemical manufacturer since 2022 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Chemical reagent >> Organic reagent >> Imide |
|---|---|
| Name | Bis-(4-tert-butylphenyl)-iodonium bis(trifluoromethylsulfonyl)imide |
| Molecular Structure | 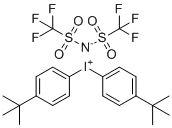 |
| Molecular Formula | C22H26F6INO4S2 |
| Molecular Weight | 673.47 |
| CAS Registry Number | 460731-32-2 |
| EC Number | 813-240-3 |
| SMILES | C1=CC(=CC=C1[I+]C2=CC=C(C=C2)C(C)(C)C)C(C)(C)C.[N-]([S](C(F)(F)F)(=O)=O)[S](C(F)(F)F)(=O)=O |
|
Bis-(4-tert-butylphenyl)-iodonium bis(trifluoromethylsulfonyl)imide is a versatile photoinitiator widely used in polymer chemistry. This compound belongs to the class of iodonium salts, known for their ability to generate reactive species under light exposure, making them effective in initiating polymerization processes. The specific structure of this compound includes two tert-butylphenyl groups attached to the iodonium center, while the bis(trifluoromethylsulfonyl)imide anion plays a crucial role in stabilizing the reactive cation, providing excellent solubility and efficiency in photoinitiation applications. The discovery of iodonium salts like Bis-(4-tert-butylphenyl)-iodonium bis(trifluoromethylsulfonyl)imide was driven by the need for efficient photoinitiators in high-performance UV-curable systems. The compound’s ability to release reactive cationic species upon exposure to UV or visible light makes it a powerful initiator for polymerization, especially in creating durable and rapid-curing materials. The introduction of tert-butyl groups enhances the solubility and performance of the compound in various organic solvents, which is essential for achieving consistent curing processes in industrial applications. In particular, the bis(trifluoromethylsulfonyl)imide anion provides excellent thermal and chemical stability, allowing the compound to remain effective under various processing conditions. This anion is large and weakly coordinating, which promotes the reactivity of the iodonium cation, ensuring fast polymerization rates. It also enhances the overall solubility of the iodonium salt in organic matrices, making it suitable for use in a wide range of photopolymer systems. One of the key applications of Bis-(4-tert-butylphenyl)-iodonium bis(trifluoromethylsulfonyl)imide is in UV-curable coatings and adhesives. These materials are widely used in industries such as automotive, electronics, and printing, where rapid curing and high durability are critical. The photoinitiator’s ability to initiate cationic polymerization under light exposure enables the production of coatings and adhesives that are resistant to chemicals, heat, and mechanical wear. Additionally, its high efficiency ensures shorter curing times, improving manufacturing productivity and reducing energy consumption. In the field of electronics, the compound is used in photoresists for the fabrication of microelectronic devices. Photoresists must be able to transfer fine patterns with high precision, and the compound’s ability to generate reactive species upon light exposure makes it an ideal choice for high-resolution photolithography. It is particularly useful in producing circuit boards and semiconductor components where accuracy and durability are essential. The biomedical sector also benefits from the application of Bis-(4-tert-butylphenyl)-iodonium bis(trifluoromethylsulfonyl)imide. In dental resins and medical device coatings, UV-curable materials require reliable photoinitiators that provide rapid curing and long-term stability. This compound meets those demands, ensuring that dental restorations and medical devices maintain their integrity over time, while reducing the curing time needed during fabrication. The synthesis of Bis-(4-tert-butylphenyl)-iodonium bis(trifluoromethylsulfonyl)imide generally involves the reaction of iodine derivatives with tert-butylphenyl compounds, followed by the introduction of the bis(trifluoromethylsulfonyl)imide anion. The compound is then purified and tested for its photoinitiating efficiency, particularly in UV-curable materials where fast polymerization is required. |
| Market Analysis Reports |
| List of Reports Available for Bis-(4-tert-butylphenyl)-iodonium bis(trifluoromethylsulfonyl)imide |