Online Database of Chemicals from Around the World
| Capot Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8558-6718 +86 13336195806 | |||
 |
capotchem@gmail.com sales@capotchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Nanjing Hengchang Biomedical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (25) 8619-9365 | |||
 |
sales@blakechem.com rongyouming@live.cn | |||
 |
QQ chat | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2011 | ||||
| Eastar Chemical Corporation | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 800-898-2436 | |||
 |
info@eastarchem.com | |||
| Chemical manufacturer since 1989 | ||||
| chemBlink standard supplier since 2014 | ||||
| Neostar United (Changzhou) Industrial Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (519) 8555-7386 +86 18015025600 | |||
 |
marketing1@neostarunited.com | |||
| Chemical distributor since 2014 | ||||
| chemBlink standard supplier since 2020 | ||||
| Changzhou Jiuwu Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (519) 8512-1086 +86 13775162768 | |||
 |
info@jiuwuchem.cn | |||
 |
QQ chat | |||
| Chemical manufacturer since 2009 | ||||
| chemBlink standard supplier since 2023 | ||||
| Matrix Scientific Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (803) 788-9494 | |||
 |
sales@matrixscientific.com | |||
| Chemical manufacturer | ||||
| Eburon Organics bvba | Belgium | Inquire | ||
|---|---|---|---|---|
 |
+32 (51) 335-068 | |||
 |
sales@eburon-organics.com | |||
| Chemical manufacturer | ||||
| Classification | Organic raw materials >> Aryl compounds |
|---|---|
| Name | 1-Bromo-2,5-dimethoxybenzene |
| Synonyms | 2-Bromo-1,4-dimethoxybenzene |
| Molecular Structure | 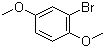 |
| Molecular Formula | C8H9BrO2 |
| Molecular Weight | 217.06 |
| CAS Registry Number | 25245-34-5 |
| EC Number | 246-756-7 |
| SMILES | COC1=CC(=C(C=C1)OC)Br |
| Density | 1.4±0.1 g/cm3, Calc.*, 1.445 g/mL (Expl.) |
|---|---|
| Index of Refraction | 1.528, Calc.*, 1.57 (Expl.) |
| Boiling Point | 269.6±0.0 ºC (760 mmHg), Calc.*, 130-131 ºC (10 mmHg) (Expl.) |
| Flash Point | 106.0±17.3 ºC, Calc.*, 110 ºC (Expl.) |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||
| |||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||
|
1-Bromo-2,5-dimethoxybenzene is an aromatic organic compound featuring a benzene ring substituted with two methoxy groups (-OCH₃) at the 2- and 5-positions, and a bromine atom at the 1-position. The molecular formula for 1-bromo-2,5-dimethoxybenzene is C8H9BrO2. This compound belongs to a class of substituted benzene derivatives, and its unique structural features make it a valuable intermediate in both synthetic organic chemistry and material science. The discovery of 1-bromo-2,5-dimethoxybenzene can be attributed to the continued exploration of functionalized aromatic compounds, particularly those involving halogenation and methoxy substitution. Such compounds are of significant interest due to their ability to undergo various chemical reactions, leading to the creation of more complex and functional molecules. The synthesis of 1-bromo-2,5-dimethoxybenzene typically involves electrophilic aromatic substitution, where a bromine atom is introduced to the benzene ring in the presence of a suitable brominating agent, such as bromine (Br₂) or NBS (N-Bromosuccinimide). The methoxy groups, which are electron-donating, make the benzene ring more reactive towards electrophilic substitution, facilitating the bromination at the 1-position. 1-Bromo-2,5-dimethoxybenzene is particularly valued in synthetic chemistry as a versatile building block for the preparation of a wide range of derivatives. The two methoxy groups on the benzene ring serve as activating groups, making the compound more reactive towards further functionalization. One of the key applications of 1-bromo-2,5-dimethoxybenzene is in the synthesis of other bioactive molecules, especially those with pharmaceutical or agrochemical potential. Its ability to undergo substitution reactions, such as nucleophilic aromatic substitution, opens up numerous pathways to introduce additional functional groups at various positions on the aromatic ring. In medicinal chemistry, derivatives of 1-bromo-2,5-dimethoxybenzene have been studied for their biological activity. The methoxy groups, which are known to increase lipophilicity and modulate the electronic properties of the aromatic ring, can play a significant role in the biological interactions of these compounds. Researchers have investigated its potential as a precursor to compounds with anti-inflammatory, anticancer, or antimicrobial activity, leveraging the reactivity of the bromine atom for the selective introduction of other pharmacophoric groups. The bromine atom in 1-bromo-2,5-dimethoxybenzene is a valuable site for cross-coupling reactions, such as Suzuki or Stille reactions, which can introduce carbon-based groups that further modify the molecule's properties. In material science, 1-bromo-2,5-dimethoxybenzene has been explored as a precursor in the synthesis of conjugated organic materials. The presence of the methoxy groups contributes to the electron-donating character of the molecule, which is advantageous for the creation of materials with desirable electronic properties. These materials have applications in organic electronics, including organic light-emitting diodes (OLEDs) and organic solar cells. The ability to functionalize the compound further enables the tuning of electronic properties, making it suitable for use in advanced electronic devices. Additionally, 1-bromo-2,5-dimethoxybenzene is used in the preparation of complex natural product analogs. For example, it has been employed in the synthesis of derivatives of the psychoactive compound 2C-H, a member of the 2C family of phenethylamines, which are of interest in the study of neurochemistry and pharmacology. The versatility of this compound as an intermediate for creating novel compounds with tailored pharmacological properties makes it a valuable reagent in synthetic organic chemistry. In conclusion, 1-bromo-2,5-dimethoxybenzene is an important compound in both synthetic organic chemistry and material science. Its role as a building block for bioactive molecules, its reactivity in cross-coupling and substitution reactions, and its potential use in organic electronics make it a compound of interest in a wide range of applications. As research continues, its applications in drug discovery and advanced materials development are likely to expand. References 2021. Intramolecular Oxidative Diaryl Coupling of Tetrasubstituted Diphenylamines for the Preparation of Bis(trifluoromethyl) Dimethyl Carbazoles. *SynOpen*, 5(4). DOI: 10.1055/a-1662-7462 2018. In Situ �Trans-Metal Trapping�: An Efficient Way to Extend the Scope of Aromatic Deprotometalation. *Synthesis*, 50(5). DOI: 10.1055/s-0036-1591953 2016. Recent Advances in Bromination of Aromatic and Heteroaromatic Compounds. *Synthesis*, 48(5). DOI: 10.1055/s-0035-1561503 |
| Market Analysis Reports |
| List of Reports Available for 1-Bromo-2,5-dimethoxybenzene |