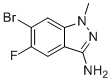
6-Bromo-5-fluoro-1-methyl-1H-indazol-3-amine is a substituted indazole derivative featuring a fused bicyclic heteroaromatic system. The compound's structure includes a 1-methylindazole core, a primary amine at the 3-position, and two halogen atoms—bromine at the 6-position and fluorine at the 5-position—attached to the benzene portion of the indazole ring system. This arrangement creates a molecule with significant synthetic utility and potential biological relevance due to its heteroaromatic nature and functional group pattern.
The synthesis of this compound typically involves multistep procedures starting from appropriately substituted hydrazines and halogenated benzoic acid derivatives. Common strategies for building the indazole core include condensation of substituted hydrazines with aromatic ketones or aldehydes followed by cyclization under acidic or thermal conditions. The methylation at the N¹-position can be achieved either during ring formation or through selective alkylation post-cyclization. Introduction of the amine group at the 3-position is generally done via nitration followed by reduction or through directed functionalization approaches.
This molecule possesses several distinct features that make it attractive in medicinal chemistry. The indazole ring is a bioisostere of indole and is commonly found in kinase inhibitors, anti-inflammatory agents, and central nervous system drugs. The primary amine at the 3-position serves as a potential point of hydrogen bonding and can be further derivatized into a wide range of analogues, such as amides, sulfonamides, or ureas, to modulate pharmacokinetics and target affinity.
The 5-fluoro and 6-bromo substitutions play a key role in tuning the electronic character of the aromatic system and influence both metabolic stability and binding interactions in biological targets. Fluorine, being highly electronegative, can impact lipophilicity and receptor binding, while the bromine atom is useful for downstream palladium-catalyzed cross-coupling reactions such as Suzuki-Miyaura or Buchwald-Hartwig amination. This makes the compound a versatile intermediate in the synthesis of more complex molecules with potential therapeutic applications.
While 6-bromo-5-fluoro-1-methyl-1H-indazol-3-amine is not a marketed drug itself, derivatives of indazole compounds bearing similar substitution patterns have been studied for activity against various biological targets, including kinases, G-protein coupled receptors, and enzymes involved in inflammatory pathways. Its structure is representative of privileged scaffolds used in drug discovery programs aimed at developing small molecule inhibitors.
In summary, 6-bromo-5-fluoro-1-methyl-1H-indazol-3-amine is a synthetically tractable and functionally rich compound. Its indazole core, combined with strategic halogenation and a reactive amine substituent, provides valuable opportunities for further chemical elaboration. The compound holds importance as an intermediate and scaffold in medicinal chemistry, particularly in the design and development of bioactive heterocyclic compounds.
|



 GHS07 Warning Details
GHS07 Warning Details