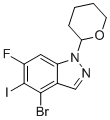
4-Bromo-6-fluoro-5-iodo-1-tetrahydropyran-2-YL-indazole is a complex heterocyclic compound that combines the indazole core structure with a tetrahydropyran-2-yl group, which is a cyclic ether. This molecule features several key structural elements: an indazole ring, which consists of a fused benzene and pyrrole ring, and a tetrahydropyran-2-yl group, a saturated six-membered ether ring attached at position 1 of the indazole. Additionally, it carries halogen atoms—bromine at position 4, fluorine at position 6, and iodine at position 5—on the aromatic ring of the indazole.
The presence of the tetrahydropyran group at position 1 introduces steric and electronic influences that can significantly affect the reactivity and biological activity of the molecule. Tetrahydropyran-2-yl groups are often employed to impart ring strain or alter the solubility and membrane permeability of organic molecules, which can be valuable in medicinal chemistry, particularly when designing molecules for oral bioavailability. The inclusion of this group can also potentially enhance interactions with biological targets by introducing flexibility to the overall structure.
The halogen substituents—bromine, fluorine, and iodine—are key features that affect the electronic distribution across the molecule, contributing to its reactivity. Fluorine is highly electronegative and can modify the molecule's dipole moment, which can affect interactions with biological macromolecules. Bromine and iodine, due to their larger atomic sizes and polarizability, are excellent candidates for metal-catalyzed reactions such as Suzuki–Miyaura or Buchwald–Hartwig couplings, making them useful for introducing further functionality or creating more complex molecules from this scaffold.
Synthesis of 4-bromo-6-fluoro-5-iodo-1-tetrahydropyran-2-YL-indazole would typically involve the preparation of a suitably substituted indazole intermediate, followed by the selective introduction of the tetrahydropyran-2-yl group at position 1. This can be achieved via nucleophilic substitution or other reactions that install the tetrahydropyran ring system onto the indazole scaffold. The halogenation of the indazole ring can be carried out using electrophilic aromatic substitution with halogenating agents like iodine monochloride (ICl), N-bromosuccinimide (NBS), and fluorinating agents such as Selectfluor.
The resulting compound, with its halogen-substituted indazole scaffold and tetrahydropyran group, could be further modified to create a range of derivatives with specific properties, particularly for pharmaceutical applications. The molecular structure suggests potential bioactivity, and compounds containing both indazole and tetrahydropyran motifs have been explored for a variety of therapeutic effects, such as anticancer, antimicrobial, and anti-inflammatory activities. Furthermore, the presence of halogens can enhance the stability and lipophilicity of the molecule, which is beneficial for optimizing drug-like properties.
In summary, 4-bromo-6-fluoro-5-iodo-1-tetrahydropyran-2-YL-indazole is a structurally rich compound with potential applications in synthetic organic chemistry and medicinal chemistry. Its halogenated indazole core combined with the tetrahydropyran group offers a versatile scaffold for the development of novel bioactive molecules. The reactivity of the halogen atoms and the steric effects of the tetrahydropyran ring make this compound an interesting intermediate for further functionalization or optimization in drug discovery.
|



 GHS07 Warning Details
GHS07 Warning Details