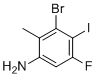
3-Bromo-5-fluoro-4-iodo-2-methylaniline is a densely halogenated aromatic amine, structurally based on a benzene ring substituted with a methyl group at position 2, an amino group at position 1 (defining it as an aniline), and three halogens—bromine at position 3, iodine at position 4, and fluorine at position 5. This particular substitution pattern creates a highly functionalized aromatic system with significant synthetic and electronic utility.
The presence of the amino group (-NH₂) on the benzene ring classifies this molecule as an aniline derivative, a class of compounds widely used as intermediates in dyes, pharmaceuticals, agrochemicals, and materials science. The methyl group at position 2 slightly increases the electron density of the ring and can influence the compound’s reactivity through hyperconjugation and inductive effects. The positioning of bromine, iodine, and fluorine in adjacent locations around the ring is chemically valuable, enabling regioselective transformations through metal-catalyzed coupling reactions.
The halogens serve distinct roles: fluorine, being small and highly electronegative, modifies the molecule’s electronic profile and can influence bioavailability in medicinal chemistry applications. Bromine and iodine, due to their larger atomic size and bond polarization, are particularly useful as leaving groups in palladium-catalyzed reactions such as Suzuki–Miyaura, Buchwald–Hartwig, and Sonogashira couplings. The selective functionalization of these sites allows chemists to introduce a variety of complex substituents with high precision, making the compound a valuable intermediate in multistep synthetic pathways.
Synthetically, 3-bromo-5-fluoro-4-iodo-2-methylaniline can be prepared through electrophilic aromatic substitution on a suitably protected aniline precursor, often requiring careful control of conditions to achieve the desired regioselectivity. Halogenation reagents such as N-bromosuccinimide (NBS), iodine monochloride (ICl), and Selectfluor or N-fluorobenzenesulfonimide (NFSI) are commonly employed. The sequence of halogenation is crucial, as the electronic influence of each substituent affects the reactivity and orientation of subsequent substitutions.
This compound's unique structure makes it highly attractive for use in the preparation of heterocyclic compounds, pharmaceuticals, and advanced materials. Aromatic amines with such substitution patterns are often incorporated into bioactive molecules due to their ability to interact with enzymes, receptors, or DNA. Moreover, halogenated anilines like this one are frequently utilized as building blocks in medicinal chemistry libraries to explore structure–activity relationships (SAR) during lead optimization.
In conclusion, 3-bromo-5-fluoro-4-iodo-2-methylaniline is a synthetically versatile and chemically rich aromatic amine, offering multiple reactive sites for functionalization. Its unique halogenation pattern and electronic properties make it a valuable intermediate for developing complex molecules in both pharmaceutical research and organic synthesis.
|



 GHS07 Warning Details
GHS07 Warning Details