Online Database of Chemicals from Around the World
| Shanghai ZaiQi Bio-Tech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 5482-4098 | |||
 |
sales1@chemzq.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink standard supplier since 2009 | ||||
| Shanghai Zhuyao Pharmaceutical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (021) 5859-6383 | |||
 |
marketing@pharmalego.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2023 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Flavors and spices >> Synthetic spice >> Lactone and oxygen-containing heterocyclic compound >> Furan and pyran |
|---|---|
| Name | 4-bromo-5-fluoro-6-nitro-1(3H)-Isobenzofuranone |
| Molecular Structure | 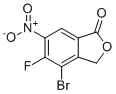 |
| Molecular Formula | C8H3BrFNO4 |
| Molecular Weight | 276.02 |
| CAS Registry Number | 2991356-61-5 |
| SMILES | C1C2=C(C(=C(C=C2C(=O)O1)[N+](=O)[O-])F)Br |
|
4-bromo-5-fluoro-6-nitro-1(3H)-Isobenzofuranone is an organic compound belonging to the class of isobenzofuranones, which are known for their diverse range of chemical and biological properties. This particular compound contains three important functional groups: a bromine atom, a fluorine atom, and a nitro group, each of which imparts specific chemical reactivity and biological activity. Its molecular structure includes a fused aromatic system that is commonly found in biologically active molecules, making it a valuable building block in synthetic organic chemistry and drug discovery. The discovery of 4-bromo-5-fluoro-6-nitro-1(3H)-Isobenzofuranone can be attributed to ongoing research into functionalized isobenzofuranone derivatives. Researchers have been particularly interested in modifying the basic isobenzofuranone structure to improve its reactivity and selectivity for certain biological targets. The introduction of halogen atoms such as bromine and fluorine, along with electron-withdrawing nitro groups, often leads to compounds with enhanced biological properties, including improved potency or selectivity for specific receptors or enzymes. The compound was likely synthesized as part of a larger effort to explore the chemical space of halogenated and nitro-substituted aromatic compounds, which are known for their diverse applications in drug design. One of the primary applications of 4-bromo-5-fluoro-6-nitro-1(3H)-Isobenzofuranone is in medicinal chemistry. The halogen and nitro substitutions play a critical role in modulating the molecule's electronic properties, which can influence its interaction with biological targets such as enzymes, receptors, and other cellular proteins. For instance, the nitro group is often used to introduce electrophilic sites in drug molecules, which can enhance reactivity with nucleophilic sites in biological systems, a property useful for targeting specific disease pathways. Similarly, the fluorine and bromine atoms can influence the compound's lipophilicity, solubility, and pharmacokinetics, making it an attractive candidate for drug development. In addition to its pharmaceutical applications, 4-bromo-5-fluoro-6-nitro-1(3H)-Isobenzofuranone also serves as an intermediate in the synthesis of other complex organic molecules. Its ability to undergo various substitution reactions, including nucleophilic aromatic substitution and electrophilic aromatic substitution, makes it a useful reagent in the preparation of more complex compounds with targeted functionalities. This reactivity allows chemists to modify the molecule further, tailoring its structure for specific applications, such as the synthesis of novel agrochemicals, dyes, or materials. The compound has also found use in the study of structure-activity relationships (SAR) in drug design. The presence of the nitro group, which is an electron-withdrawing group, along with the halogens, can significantly alter the molecule's electronic properties, making it a valuable tool for understanding how substituents on an aromatic ring can affect the binding affinity and selectivity of potential drug candidates. These studies are crucial in the development of more effective and selective therapeutic agents. In conclusion, 4-bromo-5-fluoro-6-nitro-1(3H)-Isobenzofuranone is a versatile compound with applications in medicinal chemistry, organic synthesis, and structure-activity relationship studies. Its halogenated and nitro-substituted structure offers unique reactivity and biological properties, making it an important building block for the development of new drugs and chemical reagents. |
| Market Analysis Reports |
| List of Reports Available for 4-bromo-5-fluoro-6-nitro-1(3H)-Isobenzofuranone |