Online Database of Chemicals from Around the World
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Leping Zhongsheng Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (798) 670-2188 | |||
 |
sales@zhongshengchem.com | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2008 | ||||
| Taicang Qianjing Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (512) 5364-5646 | |||
 |
sales@qjchem.com tcqxh@hotmail.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Shanghai Yingrui Biopharm Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 3358-5366 3466-6753 +86 13311639313 | |||
 |
sales02@shyrchem.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2009 | ||||
| chemBlink standard supplier since 2017 | ||||
| Nivon Specialties | India | Inquire | ||
|---|---|---|---|---|
 |
+91 9323789882 | |||
 |
nivonpharma@yahoo.com | |||
| Chemical manufacturer since 1999 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Flavors and spices >> Synthetic spice >> Lactone and oxygen-containing heterocyclic compound >> Thiazole, thiophene and pyridine |
|---|---|
| Name | 3-Chloro-4-morpholino-1,2,5-thiadiazole |
| Synonyms | 3-Chloro-4-N-morpholine-1,2,5-thiadiazole; 3-Morpholino-4-chloro-1,2,5-thiadiazole; 4-(4-Chloro-1,2,5-thiadiazol-3-yl)morpholine |
| Molecular Structure | 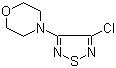 |
| Molecular Formula | C6H8ClN3OS |
| Molecular Weight | 205.67 |
| CAS Registry Number | 30165-96-9 |
| EC Number | 250-077-1 |
| SMILES | C1COCCN1C2=NSN=C2Cl |
| Density | 1.5±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 44 - 47 ºC (Expl.) |
| Boiling point | 307.8±42.0 ºC 760 mmHg (Calc.)* |
| Flash point | 139.9±27.9 ºC (Calc.)* |
| Index of refraction | 1.591 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H315-H319-H335 Details | ||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P280-P301+P317-P302+P352-P304+P340-P305+P351+P338-P319-P321-P330-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||
|
3-Chloro-4-morpholino-1,2,5-thiadiazole is a synthetic heterocyclic compound composed of a 1,2,5-thiadiazole ring substituted with a chlorine atom at the 3-position and a morpholine ring at the 4-position. Its molecular formula is C6H8ClN3OS, and the compound combines features from both sulfur- and nitrogen-containing heterocycles, which are commonly investigated for diverse biological and chemical applications. The 1,2,5-thiadiazole core is a five-membered aromatic ring containing two nitrogen atoms and one sulfur atom. This motif is known for its electronic properties and structural rigidity, making it a favorable scaffold in the development of functional materials, agrochemicals, and pharmaceuticals. The morpholine group, a six-membered ring containing both oxygen and nitrogen atoms, enhances the compound’s polarity and solubility in aqueous and organic solvents. The presence of a chloro substituent at position 3 introduces a reactive site for further derivatization or substitution reactions. The compound was synthesized as part of ongoing efforts to develop small heterocycles with potential biological activity. Thiadiazole derivatives have shown a wide range of pharmacological effects including antimicrobial, anti-inflammatory, antitumor, and anticonvulsant properties. Substituents like morpholine are often introduced to improve drug-likeness by enhancing solubility, membrane permeability, and interaction with biological targets. In medicinal chemistry research, 3-chloro-4-morpholino-1,2,5-thiadiazole and its analogs have been explored for their ability to interact with enzyme active sites or to bind receptor targets that are relevant in neuropharmacology, cancer, or infectious diseases. Although this specific compound has not been widely commercialized, closely related thiadiazole–morpholine hybrids have shown inhibitory activity against kinases, metalloproteinases, or pathogens in preclinical evaluations. Its synthesis typically involves cyclization reactions starting from thiosemicarbazide derivatives or diazonium precursors, followed by substitution with morpholine under controlled conditions. The presence of the chloro group enables further synthetic flexibility, as it can be replaced with various nucleophiles (amines, thiols, or alkoxides), allowing medicinal chemists to generate libraries of analogs for structure–activity relationship studies. Beyond pharmaceutical applications, thiadiazoles are also investigated in materials science. Their aromatic and electron-rich structures are suitable for inclusion in organic semiconductors, fluorescent dyes, and coordination complexes with interesting electronic or optical properties. The morpholine group may aid in modifying solubility or in forming hydrogen bonds in solid-state assemblies. Toxicological and environmental data specific to 3-chloro-4-morpholino-1,2,5-thiadiazole are limited, as it is primarily used in research. However, general precautions associated with halogenated heterocycles apply, and appropriate laboratory safety measures should be followed. The compound is stable under ambient conditions when stored in a dry, cool environment, away from oxidizing agents. In summary, 3-chloro-4-morpholino-1,2,5-thiadiazole is a synthetic heterocycle of interest in pharmaceutical and materials research. Its combination of the thiadiazole core, a morpholine moiety, and a reactive chloro group makes it a versatile intermediate for the development of bioactive compounds and functional materials. References 2011. High-throughput screening identification of poliovirus RNA-dependent RNA polymerase inhibitors. Antiviral Research, 91(3). DOI: 10.1016/j.antiviral.2011.06.006 2003. Electrochemical Reduction and Oxidation of 3,4-Disubstituted 1,2,5-Thiadiazoles. Russian Journal of General Chemistry, 73(5). DOI: 10.1023/a:1026155407915 2001. Synthesis of Aryloxy-Substituted 1,2,5-Thiadiazoles by the Ullmann Reaction. Russian Journal of Organic Chemistry, 37(9). DOI: 10.1023/a:1013148125019 |
| Market Analysis Reports |
| List of Reports Available for 3-Chloro-4-morpholino-1,2,5-thiadiazole |