Online Database of Chemicals from Around the World
| Epochem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 6760-1595 6760-1597 | |||
 |
seth_wang@epochem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2005 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Shaanxi Xi Yue Pharmaceutical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (029) 8823-0456 | |||
 |
hcyhsbb@163.com | |||
| Chemical manufacturer since 1998 | ||||
| chemBlink standard supplier since 2025 | ||||
| Sarchem Laboratories, Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (732) 708-1777 | |||
 |
sarchem@aol.com | |||
| Chemical manufacturer since 1984 | ||||
| Xi'an Huayao Pharm-Chem Institute | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (29) 8624-6986 | |||
 |
info@huayaochem.com | |||
| Chemical manufacturer since 1999 | ||||
| Classification | API >> Digestive system medication >> Biliary medicine |
|---|---|
| Name | N-(Hydroxymethyl)nicotinamide |
| Synonyms | 3-Pyridinecarboxylic acid N-hydroxymethylamide |
| Molecular Structure | 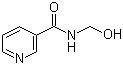 |
| Molecular Formula | C7H8N2O2 |
| Molecular Weight | 152.15 |
| CAS Registry Number | 3569-99-1 |
| EC Number | 222-668-4 |
| SMILES | C1=CC(=CN=C1)C(=O)NCO |
| Density | 1.3±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 152 - 154 ºC (Expl.) |
| Boiling point | 427.7±25.0 ºC 760 mmHg (Calc.)* |
| Flash point | 212.5±23.2 ºC (Calc.)* |
| Index of refraction | 1.569 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||
| |||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||
|
N-(Hydroxymethyl)nicotinamide is a chemically modified derivative of nicotinamide, in which a hydroxymethyl group (–CH2OH) is attached to the nitrogen atom of the nicotinamide moiety. It retains the core structure of nicotinamide, a form of vitamin B3 (niacin), and belongs to the class of substituted pyridines. This compound exists as a crystalline solid and is moderately soluble in water. Its synthesis involves the formal condensation of nicotinamide with formaldehyde, leading to the formation of an N-hydroxymethyl functional group. The development of N-(hydroxymethyl)nicotinamide was part of efforts to generate derivatives of nicotinamide with altered pharmacokinetic or functional properties. Nicotinamide itself is a key precursor of the coenzymes NAD+ (nicotinamide adenine dinucleotide) and NADP+ (nicotinamide adenine dinucleotide phosphate), which play central roles in redox reactions, energy metabolism, DNA repair, and cellular signaling. Modification of the nicotinamide structure, such as through N-alkylation or N-hydroxymethylation, has been explored to study structure-activity relationships or to develop compounds with specialized biological behavior. While N-(hydroxymethyl)nicotinamide is not a widely used pharmaceutical agent, it has been investigated in research contexts for its potential as a biochemical intermediate or modulator of nicotinamide-dependent pathways. Its structural similarity to nicotinamide suggests it may interact with enzymes or transporters related to NAD+ biosynthesis or metabolism, although detailed pharmacological data are limited. The hydroxymethyl group can potentially enhance water solubility or alter the reactivity of the molecule compared to the parent compound. The compound has been studied as an intermediate in synthetic chemistry and biochemical studies, particularly in the modification of nicotinamide analogs for use in enzyme inhibition or coenzyme mimicry. Its ability to release formaldehyde under certain conditions has also been noted, which may be relevant in the context of stability or reactivity assessments. In general, the safety, metabolism, and in vivo effects of N-(hydroxymethyl)nicotinamide have not been as thoroughly characterized as those of nicotinamide. However, no widespread toxicological concerns have been associated with its reported uses in experimental systems. Its reactivity and potential to undergo further transformations, such as oxidation or hydrolysis, are of interest in studies involving chemical biology and redox systems. In summary, N-(hydroxymethyl)nicotinamide is a synthetic derivative of nicotinamide that incorporates a hydroxymethyl substituent on the amide nitrogen. While it has not been widely applied in therapeutic contexts, it serves as a compound of interest in chemical and biochemical research due to its structural relationship to nicotinamide and potential interactions with coenzyme-related pathways. References 2010. Complexation of cyclodextrins with nicotinamide and N-(hydroxymethyl)nicotinamide in aqueous solution. Russian Journal of General Chemistry, 80(2). DOI: 10.1134/s1070363210020246 1992. Synthesis of nicotinic acid and hydroxymethylamide by the hydroformylation of 3-cyanopyridine. Pharmaceutical Chemistry Journal, 26(2). DOI: 10.1007/bf00766464 |
| Market Analysis Reports |
| List of Reports Available for N-(Hydroxymethyl)nicotinamide |