Online Database of Chemicals from Around the World
| Shanghai Zhuyao Pharmaceutical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (021) 5859-6383 | |||
 |
marketing@pharmalego.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2023 | ||||
| chemBlink standard supplier since 2024 | ||||
| Classification | Organic raw materials >> Nitrile compound |
|---|---|
| Name | 2-Bromo-3,5-difluorobenzonitrile |
| Molecular Structure | 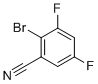 |
| Molecular Formula | C7H2BrF2N |
| Molecular Weight | 218.00 |
| CAS Registry Number | 425379-37-9 |
| SMILES | C1=C(C=C(C(=C1C#N)Br)F)F |
| Density | 1.8±0.0 g/cm3, Calc.* |
|---|---|
| Index of Refraction | 1.552, Calc.* |
| Boiling Point | 236.5±0.0 ºC (760 mmHg), Calc.* |
| Flash Point | 96.8±0.0 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H312-H315-H319-H332-H335 Details |
| Precautionary Statements | P261-P264-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P330-P332+P313-P337+P313-P362-P403+P233-P405-P501 Details |
| SDS | Available |
|
2-Bromo-3,5-difluorobenzonitrile is an organic compound that belongs to the family of halogenated benzonitriles. It features a benzene ring with bromine (Br) at the 2-position and fluorine (F) atoms at the 3- and 5-positions, along with a nitrile group (-CN) at the 1-position. The combination of halogen atoms and the nitrile group gives this compound unique chemical properties, which have led to its use in various fields, particularly in chemical synthesis and materials science. The discovery of 2-bromo-3,5-difluorobenzonitrile can be linked to the ongoing research in the synthesis of halogenated aromatic compounds with specific electronic properties. Halogenation is a well-known method used in organic chemistry to enhance the reactivity of aromatic systems, and the nitrile group further contributes to the molecule’s ability to participate in nucleophilic substitution reactions and other transformations. The introduction of fluorine atoms, known for their electron-withdrawing effects, and bromine, which is relatively reactive, plays a significant role in modulating the compound’s reactivity and stability. One of the primary applications of 2-bromo-3,5-difluorobenzonitrile is in the field of pharmaceutical and agrochemical research. The molecule can serve as an intermediate in the synthesis of various bioactive compounds. The presence of the nitrile group, along with the halogen atoms, makes this compound a versatile building block in the preparation of more complex structures. Research has shown that halogenated benzonitriles, like 2-bromo-3,5-difluorobenzonitrile, can exhibit antimicrobial, anticancer, and anti-inflammatory activities, which makes them attractive candidates for drug discovery. Additionally, 2-bromo-3,5-difluorobenzonitrile is of interest in materials science, particularly in the development of organic semiconductors and conductive materials. The fluorine atoms can help stabilize the molecular structure, while the nitrile group can enhance the material's electronic properties. These characteristics make it useful for the fabrication of organic light-emitting diodes (OLEDs), organic solar cells, and other electronic devices, where high stability and efficient charge transport are required. In the field of synthetic chemistry, 2-bromo-3,5-difluorobenzonitrile is also used in reactions such as Suzuki and Heck coupling, where it serves as a versatile reactant to form biaryl compounds and other functionalized aromatic systems. These types of reactions are essential for the synthesis of fine chemicals, agrochemicals, and various functional materials. Furthermore, 2-bromo-3,5-difluorobenzonitrile has shown promise as a precursor for designing and synthesizing new fluorinated compounds with unique reactivity profiles. The presence of both halogen atoms makes it possible to selectively modify different positions of the aromatic ring, enabling the creation of new derivatives for a wide range of applications in chemical research. In conclusion, 2-bromo-3,5-difluorobenzonitrile is a valuable compound in the fields of synthetic chemistry, pharmaceuticals, agrochemicals, and materials science. Its halogenated structure enhances its reactivity and allows for the design of novel compounds with diverse applications, from drug development to the creation of advanced electronic materials. |
| Market Analysis Reports |
| List of Reports Available for 2-Bromo-3,5-difluorobenzonitrile |