Online Database of Chemicals from Around the World
| Capot Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8558-6718 +86 13336195806 | |||
 |
capotchem@gmail.com sales@capotchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Shanghai 3s Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (021) 5290-7809 | |||
 |
market@3s-tech.net | |||
| Chemical manufacturer since 2019 | ||||
| chemBlink standard supplier since 2025 | ||||
| Chem-Impex International, Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (630) 766-2112 | |||
 |
sales@chemimpex.com | |||
| Chemical manufacturer since 1981 | ||||
| Frontier Scientific Services, Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (302) 266-6891 (888) 577-2734 | |||
 |
customerservice@frontierssi.com | |||
| Chemical manufacturer | ||||
| Oakwood Products, Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (800) 467-3386 | |||
 |
k_weaver@oakwoodchemical.com | |||
| Chemical manufacturer | ||||
| Aaron Chemistry GmbH | Germany | Inquire | ||
|---|---|---|---|---|
 |
+49 (8823) 917-521 | |||
 |
sales@aaron-chemistry.de | |||
| Chemical manufacturer | ||||
| Classification | Organic raw materials >> Hydrocarbon compounds and their derivatives >> Hydrocarbon halide |
|---|---|
| Name | 2-Chlorobenzyl bromide |
| Synonyms | alpha-Bromo-2-chlorotoluene; 1-(Bromomethyl)-2-chlorobenzene |
| Molecular Structure | 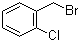 |
| Molecular Formula | C7H7BrCl |
| Molecular Weight | 206.48 |
| CAS Registry Number | 611-17-6 |
| EC Number | 210-257-2 |
| SMILES | Clc1ccccc1CBr |
| Density | 1.6±0.1 g/cm3 Calc.*, 1.583 g/mL (Expl.) |
|---|---|
| Boiling point | 235.2 ºC 760 mmHg (Calc.)*, 255.7 - 257 ºC (Expl.) |
| Flash point | 108.9 ºC (Calc.)*, 108 ºC (Expl.) |
| Index of refraction | 1.584 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |


 GHS05;GHS07;GHS09 Danger Details
GHS05;GHS07;GHS09 Danger Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H314-H335 Details | ||||||||||||||||||||||||
| Precautionary Statements | P261-P280-P305+P351+P338-P310 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Transport Information | UN 3265 | ||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||
|
2-Chlorobenzyl bromide is an organic compound consisting of a benzene ring with a chlorine atom at the 2-position (relative to the benzyl group) and a bromine atom attached to the benzyl group. It is classified as a halogenated aromatic compound and can be represented by the chemical formula C7H6BrCl. This compound is commonly used in organic synthesis and chemical research as an intermediate for the preparation of various other chemical products. The synthesis of 2-chlorobenzyl bromide typically involves the reaction of 2-chlorotoluene (a compound where a methyl group is attached to the benzene ring at the 1-position and a chlorine atom is at the 2-position) with a brominating agent such as hydrogen bromide (HBr) or a bromine reagent. The reaction proceeds through an electrophilic substitution mechanism, where the bromine atom replaces a hydrogen atom on the benzyl group, resulting in the formation of the desired product, 2-chlorobenzyl bromide. 2-Chlorobenzyl bromide is primarily used as an intermediate in the synthesis of a variety of compounds. One of the main applications of this compound is in the preparation of pharmaceuticals and agrochemicals. Its ability to undergo nucleophilic substitution reactions, where the bromine atom is replaced by a different functional group, makes it a valuable starting material in the synthesis of more complex organic molecules. In medicinal chemistry, 2-chlorobenzyl bromide can be used in the preparation of various bioactive compounds. For example, it can serve as a building block in the synthesis of benzylated compounds, which are of interest due to their potential pharmaceutical properties, such as antimicrobial, anti-inflammatory, and anticancer activities. The chlorobenzyl group can also be used as a protective or functional group in certain synthetic routes. The compound is also useful in the development of organic materials and polymers. It can be employed in the synthesis of novel materials that possess interesting properties, such as electronic conductivity, fluorescence, or enhanced chemical stability. The bromine atom in 2-chlorobenzyl bromide makes it suitable for coupling reactions, where it can react with other nucleophiles to form various polymeric or macromolecular structures. In addition to its role in organic synthesis, 2-chlorobenzyl bromide can also be employed in the preparation of dyes and other coloring agents. The chlorobenzyl group is a versatile structural element in dye chemistry, and its presence can contribute to the stability and color properties of the final dye product. Despite its usefulness, 2-chlorobenzyl bromide should be handled with care, as it is a halogenated organic compound with potential health hazards. Exposure to this compound can lead to irritation of the skin, eyes, and respiratory system. It is important to follow proper safety protocols, such as wearing protective gloves, goggles, and working in a well-ventilated area when handling the compound. Additionally, waste disposal must be conducted in accordance with environmental regulations to minimize the potential for contamination. In summary, 2-chlorobenzyl bromide is an important intermediate in organic synthesis, widely used in the production of pharmaceuticals, agrochemicals, and materials. Its ability to undergo nucleophilic substitution reactions makes it a valuable building block for the creation of more complex chemical entities. However, it should be handled with appropriate safety measures to prevent exposure to harmful effects. References 1983. 2-Chlorobenzylmercapturic acid, a metabolite of the riot control agent 2-chlorobenzylidene malononitrile (CS) in the rat. Archives of Toxicology, 54(3). DOI: 10.1007/bf01261382 2009. Bis(2-chloro-benz-yl)dimethyl-ammonium bromide. Acta Crystallographica. Section E, Structure Reports Online, 65(Pt 7). DOI: 10.1107/s160053680902159x 2015. Copper-Catalysed One-Pot Synthesis of 2,3,4,9-Tetrahydro-1H-Xanthen-1-Ones from 2-Halobenzylbromides and Cyclic-1,3-Diketones in Water. Catalysis Letters, 145(7). DOI: 10.1007/s10562-015-1538-z |
| Market Analysis Reports |
| List of Reports Available for 2-Chlorobenzyl bromide |