Online Database of Chemicals from Around the World
| Targetmol China | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (400) 820-0310 | |||
 |
sales@targetmol.cn | |||
| Chemical manufacturer since 2015 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyrimidine compound >> Aldehyde intermediate |
|---|---|
| Name | 7-Ethylpteridine-2,4-diamine |
| Molecular Structure | 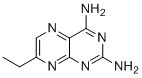 |
| Molecular Formula | C8H10N6 |
| Molecular Weight | 190.21 |
| CAS Registry Number | 80888-13-7 |
| SMILES | CCC1=CN=C2C(=NC(=NC2=N1)N)N |
| Density | 1.4±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 454.2±55.0 ºC 760 mmHg (Calc.)* |
| Flash point | 259.2±18.7 ºC (Calc.)* |
| Index of refraction | 1.749 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H335 Details |
| Precautionary Statements | P261-P264-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P337+P313-P362-P403+P233-P405-P501 Details |
| SDS | Available |
|
7-Ethylpteridine-2,4-diamine is a heterocyclic organic compound belonging to the class of pteridines, characterized by a bicyclic structure composed of a pyrimidine ring fused to a pyrazine ring. The molecule features amino groups at the 2- and 4-positions of the pteridine core and an ethyl substituent at the 7-position. Pteridines and their derivatives are widely studied due to their presence in biological systems and their role as components of cofactors such as folic acid and biopterin. The introduction of substituents, such as an ethyl group at the 7-position, allows for the exploration of structure-activity relationships and the development of analogs with potentially unique biochemical properties. 7-Ethylpteridine-2,4-diamine has been synthesized as part of research efforts aimed at understanding the chemistry of pteridine derivatives and their interactions with biological targets. Its synthesis typically involves multi-step organic reactions starting from simpler heterocyclic precursors, allowing precise modification of the pteridine nucleus. The compound is used primarily in biochemical and pharmaceutical research as a model or intermediate for the development of drugs and enzyme inhibitors targeting pathways involving pteridine cofactors. Pteridine derivatives have been implicated in processes such as nitric oxide synthesis, redox reactions, and cellular metabolism. In medicinal chemistry, analogs of pteridines including 7-ethylpteridine-2,4-diamine are investigated for their potential to modulate enzymes like dihydrofolate reductase and nitric oxide synthase, which are relevant in cancer, infectious diseases, and cardiovascular conditions. Modifications at various positions of the pteridine ring system influence binding affinity and biological activity, making compounds like 7-ethylpteridine-2,4-diamine important in structure-activity studies. From an analytical perspective, this compound can be characterized using nuclear magnetic resonance spectroscopy, mass spectrometry, and ultraviolet-visible spectroscopy to confirm its structure and purity. Its chemical stability and solubility properties depend on the substituents and the environment, factors important for its use in research settings. In summary, 7-ethylpteridine-2,4-diamine is a substituted pteridine derivative synthesized for research into the chemistry and biology of pteridine compounds. It serves as an important intermediate and tool in studying enzyme interactions and drug development related to pteridine-dependent biochemical pathways. |
| Market Analysis Reports |
| List of Reports Available for 7-Ethylpteridine-2,4-diamine |