Online Database of Chemicals from Around the World
| Shanghai Chengxi Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 18916572267 | |||
 |
chengxitechco@163.com | |||
 |
QQ chat | |||
 |
WeChat: +8618916572267 | |||
| Chemical manufacturer since 2023 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Organic raw materials >> Aryl compounds >> Biphenyl compounds |
|---|---|
| Name | Benzidine-d8 |
| Synonyms | 4-(4-amino-2,3,5,6-tetradeuteriophenyl)-2,3,5,6-tetradeuterioaniline |
| Molecular Structure | 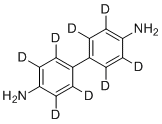 |
| Molecular Formula | C12H4D8N2 |
| Molecular Weight | 192.29 |
| CAS Registry Number | 92890-63-6 |
| EC Number | 693-875-9 |
| SMILES | [2H]C1=C(C(=C(C(=C1C2=C(C(=C(C(=C2[2H])[2H])N)[2H])[2H])[2H])[2H])N)[2H] |
| Density | 1.2±0.1 g/cm3, Calc.* |
|---|---|
| Index of Refraction | 1.667, Calc.* |
| Boiling Point | 358.7±17.0 ºC (760 mmHg), Calc.* |
| Flash Point | 203.5±20.4 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |


 GHS07;GHS08;GHS09 Danger Details
GHS07;GHS08;GHS09 Danger Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H350-H400-H410 Details | ||||||||||||||||||||||||
| Precautionary Statements | P203-P264-P270-P273-P280-P301+P317-P318-P330-P391-P405-P501 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||
|
Benzidine-d8 is the fully deuterated isotopologue of benzidine, a symmetrical aromatic diamine with the molecular formula C12D12N2. In this compound, all eight hydrogen atoms bonded to the aromatic rings of benzidine are replaced by deuterium atoms, the stable isotope of hydrogen. This substitution does not significantly alter the compound’s chemical behavior but imparts distinct characteristics in spectroscopic analysis and tracer applications due to the mass difference and nuclear properties of deuterium. The parent compound, benzidine, was first synthesized in the 19th century via the benzidine rearrangement of hydrazobenzene under acidic conditions, a classic transformation in organic chemistry. The rearrangement reaction remains the primary synthetic route for producing benzidine derivatives. Benzidine-d8 is prepared by a similar synthetic route, beginning with deuterated precursors such as hydrazobenzene-d8, which is obtained from the reduction of nitrobenzene-d5 or via direct deuteration strategies. Careful control of reaction conditions is required to ensure complete incorporation of deuterium and to prevent back-exchange with hydrogen during purification. Benzidine-d8 has specific utility in analytical chemistry, particularly in isotope dilution mass spectrometry (IDMS). In this technique, it serves as an internal standard for the quantification of benzidine in complex matrices such as environmental samples, industrial effluents, or biological fluids. The use of a deuterated analog ensures nearly identical chemical behavior with the analyte of interest while enabling differentiation in mass spectrometric detection due to the mass shift imparted by the deuterium atoms. Another established application of benzidine-d8 is in nuclear magnetic resonance (NMR) spectroscopy, where it is used to study isotope effects on chemical shifts and reaction kinetics. The substitution of hydrogen with deuterium influences vibrational modes and dipolar interactions, making it a useful tool for probing reaction mechanisms involving aromatic diamines or related systems. In mechanistic studies, benzidine-d8 may also help in tracking proton transfer or hydrogen bonding processes by eliminating signals from exchangeable protons. In the field of materials research, benzidine and its derivatives, including isotopically labeled versions, have been investigated for their role in organic semiconductor materials. While benzidine itself is less commonly used in modern electronic applications due to safety concerns, its structure serves as a model for understanding charge transport in extended conjugated systems. Deuterated analogs like benzidine-d8 can be employed in fundamental studies that involve neutron scattering, where the use of deuterium enhances contrast due to its neutron-scattering properties. Benzidine-d8 also contributes to toxicological studies and environmental monitoring. As benzidine is a known human carcinogen and historically used in the dye industry, trace analysis of this compound and its metabolites is crucial for occupational safety and environmental protection. Using benzidine-d8 as a reference standard ensures high accuracy in detecting low levels of benzidine and distinguishing it from structurally similar compounds or degradation products. All applications of benzidine-d8 require strict laboratory handling protocols, as the toxicological profile of the compound is assumed to be similar to that of its non-deuterated counterpart. Despite the substitution with deuterium, its aromatic amine functionality and associated health hazards remain a concern. Therefore, procedures involving benzidine-d8 must comply with safety regulations pertaining to carcinogenic aromatic amines. The scientific use of benzidine-d8 illustrates the broader role of isotopically labeled compounds in analytical and mechanistic chemistry. Its stable isotope labeling provides precise tools for quantification, structural elucidation, and mechanistic interpretation, especially in areas where trace-level detection and discrimination between isomers or analogs are essential. The integration of benzidine-d8 into validated analytical methods and controlled studies underscores its relevance across multiple disciplines of chemical research. References 2024. Cell membrane chromatography relative competitive method for the accurate determination of relative KD values of drug-receptor interactions. Archives of Pharmacal Research, 47. DOI: 10.1007/s12272-024-01525-x 2023. Molecular Model of Norfloxacin Translocation through Yersinia pseudotuberculosis Porin OmpF Channel: Electrophysiological and Molecular Modeling Study. Biochemistry (Moscow), Supplement Series A: Membrane and Cell Biology, 17. DOI: 10.1134/s1990747823070024 2019. Fluoroquinolones structural and medicinal developments (2013�2018): Where are we now? Bioorganic & Medicinal Chemistry, 27(14). DOI: 10.1016/j.bmc.2019.05.038 |
| Market Analysis Reports |
| List of Reports Available for Benzidine-d8 |