Online Database of Chemicals from Around the World
| Shanghai ZaiQi Bio-Tech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 5482-4098 | |||
 |
sales1@chemzq.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink standard supplier since 2009 | ||||
| Classification | Organic raw materials >> Heterocyclic compound >> Piperidines |
|---|---|
| Name | tert-Butyl 4-((4-(3-aminophenyl)piperazin-1-yl)methyl)piperidine-1-carboxylate |
| Molecular Structure | 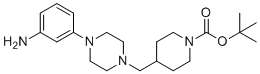 |
| Molecular Formula | C21H34N4O2 |
| Molecular Weight | 374.52 |
| CAS Registry Number | 2636798-59-7 |
| SMILES | CC(C)(C)OC(=O)N1CCC(CN2CCN(c3cccc(N)c3)CC2)CC1 |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319 Details |
| Precautionary Statements | P501-P270-P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313-P301+P312+P330 Details |
| SDS | Available |
|
tert-Butyl 4-((4-(3-aminophenyl)piperazin-1-yl)methyl)piperidine-1-carboxylate is a multifunctional, protected heterocyclic building block that combines a piperidine ring, a piperazine moiety, and a pendant aryl amine group. Its structure features a piperidine core with a tert-butoxycarbonyl (Boc) protecting group on the 1-nitrogen, a methylene linkage to the piperazine, and the piperazine is further substituted with a phenyl ring bearing a 3-amino substituent. This compound is primarily used in medicinal and synthetic organic chemistry as an intermediate. The Boc on the piperidine nitrogen confers stability during synthesis and can later be removed under acidic conditions to liberate the free amine. The piperazine provides a flexible secondary amine scaffold that can be functionalized or linked further. The 3-aminophenyl substituent offers a handle for additional chemistry: it can participate in coupling reactions, be derivatized to more complex pharmacophores, or provide hydrogen-bonding interactions in drug design. In drug-discovery contexts, such an intermediate is valuable for constructing libraries of ligands, inhibitors, or linker regions in bifunctional molecules (e.g., PROTACs). After deprotection and further functionalization, it allows medicinal chemists to append additional pharmacophores, optimize binding interactions, or modulate physicochemical properties like solubility and lipophilicity. Synthesis of this molecule would typically begin with Boc-protected 4-(chloromethyl)piperidine or a similar precursor, which is then alkylated with a piperazine derivative bearing the 3-aminophenyl substituent. Alternatively, one might first build the piperazine–phenyl structure and then connect it via reductive amination or nucleophilic substitution to the piperidine core, followed by installation of the Boc protecting group if not already present. From a practical standpoint, care should be taken when handling this compound. The Boc group is acid-labile, so it needs to be stored under conditions that avoid strong acids. The amino group on the phenyl ring can react under typical coupling or derivatization conditions, so protecting-group strategies may be required, depending on downstream chemistry. Overall, tert-Butyl 4-((4-(3-aminophenyl)piperazin-1-yl)methyl)piperidine-1-carboxylate is a strategically designed intermediate in medicinal chemistry, combining protective group chemistry with synthetic versatility to enable the construction of diverse, biologically active molecules. |
| Market Analysis Reports |
| List of Reports Available for tert-Butyl 4-((4-(3-aminophenyl)piperazin-1-yl)methyl)piperidine-1-carboxylate |